
OAT
Interpretation of OAT data
Data represented in the Mosaic (formerly Great Plains) Organic Acids Test, or similar tests by Genova are represented in reference to the average ranges for data obtained by these laboratories. The data is normalized and an arbitrary range that covers 90% of the data is assigned a normal value. The value, though, does not represent clinical normality, it represents the ranges that are normally seen in the laboratory. The ranges are also not ranges that are assigned to any particular condition, such as "normal for a person with autism", or "normal for a person with diabetes", or "normal for a person with Chronic Fatigue Syndrome". Further, the ranges that these laboratories measure are most likely the ranges for persons who have some sort of medical condition that warrants the expense that is involved in testing. As such, the ranges, almost by definition. do not include persons who are biochemically normal. In order to get biochemically normal data, it is essential that data be obtained from persons who are healthy, who have no predisposing condition, eat a well balanced diet, and are of normal weight. Hence data should represent Optimal, Desirable, Average, and Deficient. Pathology labs, though represent data as part of a "normal distribution", rather than Optimal or Desirable, and so generally Average and Deficient are more likely to fit into the "normal distribution", as these are generally those who are being tested.
The Physician's Trap
Whilst the comments on Interpretation of OAT data, is inherently obvious, it does not appear to be in general parlance, thus, the majority of Physicians are "trapped" into using the ranges as defined by GPL and other labs, as being normal, hence many conditions with abnormal biochemistry are missed, and the date dismissed as being "normal". However, for many of the ranges as represented in the OAT, biochemically normal data is out of range low. Hence for many people they are MISDIAGNOSED due to the IGNORANCE of the Medical Profession.
Changes with Time
As the population changes, the normal distribution of data changes. Hence if one does not have historical data, then if you used the ranges as defined by the pathologists, then the measured range could appear "normal" as defined by the Path Measurements, however it is not metabolically normal. For instance in the Metabolites measured below, metabolically normal HVA is 0.49-1,0, VMA 0.72 - 1.0, 5HIAA < 1.0, QA 0.48-1.0 and KA 0.5-1.0. Hence in the sample the person is actually high in HVA, VMA, 5HIAA, QA and KA. Yet would be deemed to be deficient in Neurotransmitter metabolites (a sign of Methyl B12 sufficiency), yes is actually high in Neurotransmitter metabolites (a sign of Methyl B12 insufficiency). As the incidence of Autism, ADHD, CFS, depression, AD, PD and OCD go up, the "normality" will shift, and so the B12 deficiency will not be recognized. The consequences of this are dire, as functional B12 deficiency conditions will be missed. Examples are given below. In 2015 the supposed normal range was HVA 0.39-2.2, VMA 0.53-2.2, 5HIAA <2.9, QA 0.52- 2.4, KA 0.12-1.8. As you can see from the data, the biochemical "normality" was actually off range low. In 2022 the ranges were very similar, however by 2025, the ranges had shifted dramatically higher HVA 0.49-13, VMA 0.72 - 6.4, 5HIAA <11, QA 0.48-8.8, and KA 4.2. Of note is that 2025 data the HVA is deemed to be out-of-range low, yet in 2015, it would have been out of range high!! Similarly with VMA and QA.
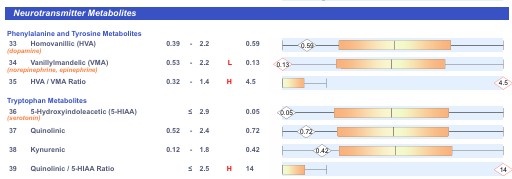
Neurotransmitters 2015

Neurotransmitters 2025
Paralleling the change in ranges for the neurotransmitters, there has also been a change in oxalate markers, glycolytic markers, and Krebs cycle markers, particularly B2, B1 and iron deficiency markers between 2015 (top) and 2023 (bottom). Hence the range for oxalate has increased from 8.9-67 to 6.8 - 101, lactate 0.74 - 19 to <48, and there has been a huge increase in the functional iron marker, citrate (an aconitase activity marker) from 2.2 - 260 to <507. The breadth of the standard range has also increased significantly with much data exceeding the ranges.


Estimation of iron sufficiency
Determination of iron sufficiency by Pathology Laboratories would be one of THE classic "Physicians Traps". This can be readily seen when one looks at ranges for ferritin in different communities. Thus, the average male vegetarian has a serum ferritin range of 30-75 ug/L, with the average female vegetarian with a range 11-35 ug/L. Most pathology labs will not flag iron deficiency until ferritin levels are 12-15 ug/L. This though is in distinct contrast to biochemical normality. Here it can be seen that biochemical signs of iron deficiency start at 60-70 ug/L, when the enzyme aconitase starts to uncouple and becomes an iron sensing molecule. At this stage the energy entering Krebs cycle is compromised and there is a linear increase in "waste" citrate, which parallels the linear decrease in mini mental score estimation as ferritin drops below 60. Clearly then, these ranges whilst "Typical" for vegans, are not biochemically normal. Similar ferritin levels are found on Lacto-ovo vegetarians, however, the average ferritin for meat eaters is in the range 100-200 ug/L. Measurement of aconitase activity in this range shows 100% activity, and further there is no reduction in serum haemoglobin or cellular haematocrit. In contrast, there is a drop in haemoglobulin from the normal range of 14.6 - 16.0, to an oxygen carrying deficiency of 10.9 to 12.5 in the vegan population. Hence, whilst these ranges may be deemed "Normal" by pathology laboratories, they are by no means biochemically normal. Curiously, the biological consequences of low ferritin are not discussed in communities such as the vegan communities, however terminology such as "normal for a vegan" is common. Hence we have the concept of a "Normal Range of ferritin for a Vegetarian male (30-75 ug/L) and a vegetarian female 11-35 ug/L, BUT, neither of these ranges represent biochemical normality. This is part of the physician's trap. Hence if the medical profession does not know what biochemical normality is, then even simple assessments such as the biochemically normal range for iron will be lost on the physician. To make this worse, the physician is "ruled" by their local medical association, and so is not able to "Think outside the box" on these matters but is forced to Toe-the-line, as far as assessment. This means that a person deemed to be sufficient in one country can be totally deficient in another. CLEARLY THIS IS LUDICROUS.
Representative measured ranges for ferritin
Sydney Australia 30-400 ug/L - Laverty's Pathology Gold Coast 30-200 ug/L Melbourne Australia 20-200 ug/L SEALS pathology 10-100 ug/L, QML Pathology 20-140 ug/L. SNP PathologyQld 30-200 ug/L
UK 13-200 ug/L
Ferndale WA, USA 6-170 ug/L 15-150 ug/L Labcorp
Sacramento CA, USA 14-80 ug/L
Canada 12-70 ug/L This various from state to state Ontario 12-100 ug/L
Bulgaria 14-150 ug/L
Poland mean 34.8 (Range 2.7- 135.2) female athletes; Malczewska-Lenczowska etal, 2018)
As can be seen even within the USA the ranges vary from state to state, with a big variation from country to country. Similarly in Australia. Clearly, these ranges, represent the average ranges for the populations measured. technically biochemical deficiency in iron can be seen in the bone marrow when ferritin is less than 100 ug/L and elevations in citrate occur when ferritin is less than 60. Under the guidelines in Sacramento and Canada, everyone is biochemically deficient in iron.
Ranges for Haemoglobin are also highly variable from place to place and day to day even from the same lab. They bare little correlation with sufficiency, rather will the range of data seen by the Pathology labs. Interestingly, Pathology Labs appear to be more simple analytical labs, rather than true laboratories representing pathology.
Sydney Australia 130-180; 115- 165; Melbourne Australia 105-135
UK 120-160 ug/L
VA USA 11.1-15.9, NJ 10.9-14.8
Ontario Canada 112-141
Bulgaria 110-147
Poland Mean 135 (Range 116-134 female athletes; Malczewska-Lenczowska etal, 2018)
As too Haematocrit
Sydney Australia 0.4 -0.54; 0.34 - 0.47 Melbourne Australia 0.29-0.4
UK 0.36-0.48
VA USA 0.34 - 0,466 NJ 0.32 - 0,433
Ontario Canada 0.343 - 0.426
Bulgaria 0.31- 0.41
Poland 40.3 (Range 34.7 -- 45.3 female athletes; Malczewska-Lenczowska etal, 2018)
Apart from the reduction in the activity of the Krebs cycle enzyme aconitase as ferritin drops below 60-70 ug/L, evidence of clinical abnormality of ferritin levels below 75 is seen in the increased incidence of Restless Leg Syndrome (Allen etal, 2018, Dye etal, 2017), and an increased risk of acute renal failure after cardiopulmonary bypass is seen in patients with ferritin levels below 130 ug/L (Davis etal, 1999). Reduced aconitase activity can be determined from the OAT value for citric acid. Biochemical normality is around 100, however exceedingly high values are seen in many children with ASD. The higher the value for citrate the lower the activity of aconitase, and the lower the mini mental score estimation see https://b12oils.com/iron.htm
Haemoglobin is one of the standard measurements of iron sufficiency, and as iron levels drop, so too does Haemoglobin. In iron sufficiency, Haemoglobin is 14.5 mg, yet the majority of Pathology Labs either do not include this in the "normal" range of Hb See above. Hence the majority of data measured by this lab is technically iron deficient. A typical example according to Dynacare Plus
There are four markers that should agree with each other, in order to make a proper diagnosis of iron sufficiency.
1. Haemoglobin. Sufficiency of iron is seen when Hb levels are greater than 144 g/L (14.5 g/dl). Levels can be above this in those who live in a high altitude. Once Hb levels drop below 14.5 iron insufficiency results. Given that Hb is responsible for carrying oxygen, insufficiency in O2 max will occur as Hb drops, this is despite sex, male or female.
2. Haematocrit. Sufficiency of iron is seen when Hct levels are greater than 0.45 (45%). Normally this tracks very closely with Hb
3. Ferritin. Sufficiency of ferritin starts when ferritin is above 100 ug/L (100 ng/ml). As levels drop below 100 changes can be seen in the bone marrow, suggesting biochemical deficiency. In the OAT changes can be seen to the level of citrate when ferritin drops below 60 ug/L, readily demonstrating biochemical insufficiency.
4. Citrate. Citrate is the substrate for the iron-sulphur enzyme, aconitase. When ferritin drops below 70, then enzyme starts to uncouple and so less citrate is metabolized leading to an increase in citrate. This increase represents biochemical insufficiency of iron, but it also indicates an inefficiency of energy metabolism, such that the higher the value the greater the energy loss. One could think of this as an indication of how well the body has "tuned" the citric acid cycle. Iron insufficiency can therefore be suspected when citrate rises above 150 in OAT.
IF the markers do not agree, then there is a problem. Hence in a prolonged COVID infection, ferritin in serum rises dramatically, but Hb/Hct/citrate all rise, hence in this case iron deficiency is seen in synthesis of Hb/Hct and in the activity of aconitase.
Hypothyroidism
Another area of concern is the definition of hypothyroidism as determined by pathology labs. Data for euthyroid in many labs states a range of 0.39 to 4.6 mIU/L. This, though varies from lab to lab. Comparison with OAT data though, shows that hypothyroidism as defined by elevation in appropriate organic acids - and particularly glutaric acid, seems to start at a range above 1.4 mIU/L. Despite this, Labs as recommended by the NHS in the UK are still quoting TSH levels of 0.34-5.6 miu/L as being normal. Another case of misdiagnosis of hypothyroidism, due to the Pathology Lab and sanctioned by the NHS, No wonder they do not think that Iodine deficiency is a problem in the UK!!
Serum vitamin B12
Serum vitamin B12 is almost useless as a marker, unless it is low, in which case it indicates an absolute deficiency. This should be mirrored by markers of iron deficiency (Hb/Hct/ferritin). If serum B12 is high, and yet iron markers are low, then this is "suspicious" and further investigation is warranted. The elevated serum B12 (>450 pmol/L) is generally indicative of paradoxical B12 deficiency, BUT, lower values can also be indicative of this, hence secondary markers are required. Once again, ranges for serum B12 vary depending upon where they are taken. Once again, the values are highly variable even within labs.
Sydney, NSW Australia 300-700 pmol/L Lafertties - Feb, 2022
Sydney NSW Australia 160-740 Laferties May, 2024
Dubai 300-1200 pmol/L
Bulgaria 133-675 pmol/L
Portugal 196-675 pmol/L
Active B12 measurements are extremely confusing and are not at all understood by the physician. Hence "Active B12" technically means the amount of transcobalamin that has some analogue of B12 bound to it. The analogue could be Adenosyl, Methyl, hydroxyl, cyano, Co(II), Co(I) or GSH, or any one of over 50 vitamin B12 analogues of which only Adenosyl and Methyl are biologically active. This is not understood by the clinician and the test should be scrapped.
Adenosylcobalamin deficiency markers
MMA
MMA (methylmalonic acid) is one of the main determinants of Adenosyl B12 deficiency outlined in the OAT. As AdenosylB12 deficiency increases, so too does the level of MMA. Hence by definition in a vitamin B12 replete person MMA should be as low as possible.
Ranges in OAT have been established as
Normal 0.55 +/- 0.15: Autism 2.1 +/- 1.4: CFS 1.33 +/- 0.89; GPL < 5.2, range 1.0 - 4.0
In this example one can see that "normal B12 sufficient" persons sit outside the entire supposedly normal range for MMA, and that all persons with CFS and ASD would have been judged as normal, according to GPL. The reality is, though, that all persons with autism and CFS are B12 deficient, by this and many other markers. As such under the "Physician's Trap" scenario, neither group would be treated with vitamin B12, nor the cause of their conditions assigned to B12 deficiency.
Other Adenosylcobalamin deficiency markers
Elevated levels of branched chain amino acids, and odd chain fatty acids - are signs of deficiency of the Adenosyl-B12-dependent enzyme MMA-CoA mutase. Elevations can be seen for the plasma amino acids Isoleucine, leucine, and Valine. In OAT there are elevations in ethylmalonate, and methylsuccinate.
Methylcobalamin deficiency markers
The standard serum marker for Methylcobalamin deficiency is elevations in homocysteine. This is normally accompanied by low creatinine levels.
In OAT, there are many markers including HVA, VMA, QA, KA, 5HIAA, pyroglutamic acid, and 3-hydroxymethylglutaric acid (HMG).
Creatinine - a surrogate B12 deficiency marker
Creatinine comes from the break-down of creatine, and hence very low creatine values are indicative of functional B12 deficiency markers.
Here
again the ranges change from lab to lab.
Normal range for creatine is 60-110 ug/L, BUT, SEALS pathology (Australia) has
20-44. Hence their whole population is B12 deficient.
Paradoxical vitamin B12 deficiency
A feature of Paradoxical B12 deficiency is elevated B12 levels in serum. The incidence of Paradoxical B12 is so frequent the the "normal range" measures by Pathology laboratories has greatly distorted the range for serum B12, hence OAT markers such as MMA and other B12 deficiency markers, such as HVA/VMA, QA, KA, and 5HIAA, and pyroglutamate (a product from deficiencies in the sulphation pathway) must be used to establish normality. A typical example according to Dynacare Plus. Paradoxical B12 deficiency is particularly common in ASD, CFS, PD and AD. Examples can be seen at https://b12oils.com/b12.htm and in the publication on vitamin B12 deficiency in autism. Normal ranges are to be found in the publication.
We have created a link
to an excel spreadsheet in which one can insert data such as OAT, Hb, Hct, TSH,
etc and obtain a read-out on insufficiency. Data has been obtained from over
2000 individuals and relative values plotted against various markers. See
OATanalysis
Evaluation of OAT data
Functional vitamin B2 deficiency and Glutaric acid
One commonly used marker in the OAT is the level of glutaric acid, with increasing levels of glutaric acid being indicative of functional B2 deficiency, as such the lower the glutaric acid marker the greater the amount of functional B2. Increased glutaric acid is indicative of functional B2 deficiency and a reduced ability to burn fat for energy.
Ranges in OAT have been established as
Normal 0.27 +/- 0.24: Autism 1.25 +/- 1.77
Functional B2 deficiency can be seen in the OAT, by elevations in adipic acid, oxalic acid, lactic acid, succinic acid, and other markers. See https://b12oils.com/b2.htm Examples can be seen in the publication on vitamin B2 deficiency in autism.
Activation of vitamin B2 (riboflavin) occurs in two steps.
Step 1. Phosphorylation of riboflavin to form flavin-mononucleotide (FMN). This step requires adequate Iodine and Selenium. Lack of Iodine and/or Selenium can be determined by the increase in succinic acid (>3.5) and increase in the QA:KA ratio (see the article on vitamin B12.
Step 2. Riboflavin kinase (a molybdopterin enzyme) converts FMN to FAD. If glutaric acid levels are low, and succinate <3.5 and QA:KA <3.0, yet other B2 deficiency markers are elevated, Molybdenum deficiency is likely
Testing TSH/T4/T3
In Australia tests can be ordered from https://imedical.com.au
Functional vitamin B2 deficiency and Porphyria
The last step in closing the porphyrin ring during the synthesis of the heme ,molecule involves an FAD-dependent enzyme, (TBA), which reduces protoporphyrin IX . In functional B2 deficiency the ring cannot be closed, and the sequestered iron precipitates, and incomplete porphyrin ring, leading to porphyria.
In the UK there is an EGRA test for functional B2.
Shifting "Normality" - HMTA example
Two commonly assessed markers in the HMTA are the levels of Selenium (essential for conversion of T4 to T3) and Molybdenum (essential for the conversion of FMN to FAD - two active forms of vitamin B2). Over time, soils in many parts of the world have become more and more depleted in Selenium and Molybdenum, with the result that the "normal ranges" for these two metals has been slowly reducing. Formerly the range for Selenium was 0.7 - 1.1, however this has drifted slightly lower to 0.55 - 1.1. Around 50% of children with autism have HMTA of <0.7.
Similarly, there has been a shift in Molybdenum ranges, which is more dramatic. Formerly the range was 0.05 - 0.13, however, over the last 5 years, there has been a dramatic drop in Molybdenum levels, such that the range has now moved to 0.02 - 0.05. Around 75% of children with autism have Molybdenum that is <0.05.
The shift away from dairy products and the consumption of alternatives such as soy and almond drinks has also resulted in an alarming drop in the ranges for calcium, which 10 years or so ago were 300-1200 ppm, but in many areas the range is now as low as 125- 350 ppm.
Variability in OAT analysis
Whilst it is generally assumed that the ranges in assays such as the OAT would be very constant, and so the definitions of normality would be the same from day to day, this is definitely not the case. See the two OAT examples below. In the upper example the range for HVA is 0.49-13, and the data point "3.0" is out of range low (as per the graph). In the lower example, the range for HVA is 0.39 - 2.2, and the data point "2.2" is in the upper range of "normal". So depending upon the day of assessment, a person could be out of range high or low (3.0), or in range (2.2). Clearly this is ludicrous, BUT, it is very, very common in the data from Pathology Labs.


Energy loss in OAT
By definition the urinary Organic Acids Test represents the measurement of excretory organic acids, many of which represent ingested calories, that have been processed in the body in an attempt to generate energy from them, and due to nutritional deficiency are wasted/excreted into urine. Examples of energy loss are elevations in oxalate, glycolate, lactate, and citrate. As such the higher the values for these markers the greater the energy loss.
Summary
Data from pathology labs appears to be reliably accurate, however the ranges that are defined are determined by the clients getting the analysis done and as such do not represent metabolically normal ranges. As such, it is important to have data from metabolically normal individuals in order to ascertain abnormal values. The data from the assays, can though be used to monitor change, and once metabolic normality has been defined is very useful in establishing supplement regimes.
References
Position of the Academy of Nutrition and Dietetics: Vegetarian
Diets. J Acad Diet Nutr. 2016;116:1970-1980.
Davey GK, Spencer EA, Appleby PN, Allen NE, Knox KH, Key TJ. EPIC-Oxford:
lifestyle characteristics and nutrient intakes in a cohort of 33 883 meat-eaters
and 31 546 non meat-eaters in the UK. Public Health Nutr. 2003 May;6(3):259-69.
Haddad EH, Berk LS, Kettering JD, Hubbard RW, Peters WR. Dietary intake and
biochemical, hematologic, and immune status of vegans compared with
nonvegetarians. Am J Clin Nutr. 1999 Sep;70(3 Suppl):586S-593S.
Alexander D, Ball MJ, Mann J. Nutrient intake and haematological status of
vegetarians and age-sex matched omnivores. Eur J Clin Nutr. 1994
Aµg;48(8):538-46.
Obeid R, Geisel J, Schorr H, Hübner U, Herrmann W. The impact of vegetarianism
on some haematological parameters. Eur J Haematol. 2002 Nov-Dec;69(5-6):275-9.
Harvey LJ, Armah CN, Dainty JR, Foxall RJ, John Lewis D, Langford NJ,
Fairweather-Tait SJ. Impact of menstrual blood loss and diet on iron deficiency
among women in the UK. Br J Nutr. 2005 Oct;94(4):557-64.
Reddy S, Sanders TA. Haematological studies on pre-menopausal Indian and
Caucasian vegetarians compared with Caucasian omnivores. Br J Nutr. 1990
Sep;64(2):331-8.
Worthington-Roberts BS, Breskin MW, Monsen ER. Iron status of premenopausal
women in a university community and its relationship to habitual dietary sources
of protein. Am J Clin Nutr. 1988 Feb;47(2):275-9.
Waldmann A, Koschizke JW, Leitzmann C, Hahn A. Dietary iron intake and iron
status of German female vegans: results of the German vegan study. Ann Nutr
Metab. 2004;48(2):103-8. Epub 2004 Feb 25.
Allen etal. Evidence-based and consensus clinical practice guidelines for the iron treatment of restless leg syndrome.... Sleep Med. 2018 41: 27-44
Dye etal Outcomes of long-term iron supplementation in pediatric restless leg syndrome....Sleep Med 2017 32:213-219
David etal. Acute renal failure after cardiopulmonary bypass is related to decreased serum ferritin levels J Am Soc Nephrol. 1999 10: 2396-402)
Malczewska-Lenczowska J, Sitkowski D, Surała O, Orysiak J, Szczepańska B, Witek
K. The Association between Iron and Vitamin D Status in Female Elite Athletes.
Nutrients. 2018 Jan 31;10(2):167. doi: 10.3390/nu10020167. PMID: 29385099; PMCID:
PMC5852743.
Copyright © 2018 B12 Oils. All Rights Reserved.
Reproduction in whole or in part in any form or medium without express written
permission is prohibited