
Iron Deficiency in Autism
-
Iron deficiency is the most prevalent micronutrient deficiency in the world, affecting about 2 billion people, particularly mothers and children
-
Iron deficiency is the primary cause of anaemia, affecting roughly one-quarter of the world's population.
-
Iron deficiency is the second most preventable cause of mental retardation in the world.
-
Iron deficiency during pregnancy leads to iron deficiency in the neonate
-
Biochemical iron deficiency is extremely common in pre-menopausal women
-
Iron deficiency during pregnancy increases the risk for preterm labour, low birth weight and increased infant mortality.
-
Iron deficiency in children is associated with developmental delay and behavioural disorders
-
Iron deficiency in children is associated with lower verbal intelligence, attention and concept learning
-
iron deficiency has been associated with lower vitamin D status
-
More than 80% of children with ASD have been found to be iron deficient at time of assessment
-
Iron deficiency has been associated with epilepsy in ASD
-
Iron deficiency is associated with hypotonia and is a Red Flag for developmental delay
Nutritional Sufficiency of Children
The maternal decision to carry a child to term creates a beneficence-based fiduciary obligation on the part of the mother (and physician) to act in the best interest of the unborn child, and to sacrificially care for and nurture that child, There can be no doubt that this extends to ensuring nutritional sufficiency of the child in its early life. (Centre for Bioethics). In order for mother to do this, the mother must ensure that she is nutritionally sufficient before conception of the child.
Role of iron in brain development
Iron loading of the brain, occurs predominantly in the last trimester of foetal growth. The brain is highly susceptible to iron deficiency during the late foetal and early neonatal time period. Deficiency at this time is associated with altered expression of genes critical for development and function, and deficiency at this time causes neurocognitive dysfunction, impaired cognition, and altered social behaviour, reduced synaptic plasticity, myelination and synthesis of neurotransmitters, which may continue even after iron stores have become replete (Ferrierr etal, 2019; Georgieff et al, 2019).
Iron deficiency in the mothers results in decreased oxygen transport, which decreases as the haematocrit and haemoglobulin concentration of the mother's blood decreases. This is then further exacerbated by reduced iron in the foetus, leading to further reduction in oxygen transport across the placenta and reduced availability of oxygen to the developing foetus due to reductions in foetal haemoglobin and foetal haematocrit.
Iron deficiency has been associated with poorer voice recognition in the neonate, and whilst infants with iron sufficiency can be shown electrophysiologically to recognize their mother's voice, children with fetal-neonatal iron deficiency did not. This was associated with poorer auditory recognition memory at 2 months of age and is consistent with effects of iron deficiency on the developing hippocampus in the brain.
Iron deficiency in utero affects the development of cerebellar Purkinje cells, specific neurons involved in fine-tuned motor control, balance, proprioception, and the vestibular-ocular reflex (VOR), a reflex essential in eye-tracking of an object when the head is moved. Several reports have indicated that there are 35-95% fewer Purkinje cells in the cerebrum of ASD brains in comparison to neurotypically normal brains. Damage to this area of the brain has been associated with a range of conditions including ataxia, intention tremors, stiff or high stepping gait, lack of awareness of foot position and a general inability to judge distance and space. Reduced VOR has implications for reading and focusing and as such causes problems in focusing and reading of fine print.
Iron deficiency has been associated with lower production of brain-derived neurotrophic factor, an important factor involved in the development of learning, memory and behaviour (Yusrawati etal, 2018)
Decreased iron concentration in the brain is associated with irritability, apathy reduced ability to concentrate and with various other deficiencies in cognition. Iron deficiency in the brain is also associated with deficit in language capability. In addition, Iron deficiency is associated with hypomyelination of nerves, thus reducing the maturation of rapid impulse transmission along nerves.
Iron deficiency also correlates with a decreased nerve conduction velocity (Kabakus etal, 2002), and reduced energy production in Krebs cycle, with the result that decreases in iron are associated with decreased mini mental score a measure of IQ (Mangialasche etal, 2015). Despite the known risks of iron deficiency in utero, a recent study in Europe (Milman et al, 2017) revealed that only 20-35% of European women of reproductive age had sufficient iron stores (SF concentration >70 ug/L) to go through pregnancy without supplementing iron. The mean serum ferritin in women in the study was only 26-38 ug/L. Given that it is known how critical iron sufficiency is for the developing embryo and neonate, it beggars the question that if as a result of this iron deficiency, the mother gives birth to a child with developmental delay, why should society subsidize these children through systems such as the National Disability Insurance Scheme (Australia and United Kingdom), the Social Security Disability (USA) or the Child Disability Benefit (Canada). Despite the huge economic costs of iron deficiency and its effect on the embryo and new born child, “The U.S. Preventive Services Task Force found insufficient evidence to recommend screening or treating pregnant women for iron deficiency anemia to improve maternal or neonatal outcomes. “ (Wang 2016).
Iron Deficiency in the fetus
Iron deficiency is very common in pregnancies (40-50% as determined by IDA), however, not all iron mothers with ID have children with ASD. Further the difference in iron levels in the serum of kids +/- ASD is very little. Low iron intake, when combined with advanced age of the mothers, resulted in a five-fold increased risk of having an ASD child (Schmidt 2014).
Brain accumulation of iron appears to happen primarily in utero, and uptake of iron into the brain, post weaning, is limited (63). It is critical that the developing foetus receives sufficient iron for neuronal development in the brain and that there is sufficient iron for the neonate to have adequate stores to last for the first six months of life. This is because the immature neonatal gut is not developmentally mature and as such cannot regulate the uptake of iron (Radlowsky and Johnson 2013), and additionally breast milk is very low in iron content. Maturation of the gut will be further compromised if the mother is vitamin B12 deficient as melatonin production is reduced in B12 deficiency and melatonin secreted by the mammary gland is required for gut maturation. The majority of the fetal liver stores (66%) are acquired in the last one-third of pregnancy and so infants born prematurely with a low birth weight are at greater risk of iron deficiency. Infants who are born to iron deficient mothers are still found to be abnormally low in iron 9 months after birth, even if provided adequate dietary iron (Radlowsky and Johnson 2013). Iron deficiency at this time creates other problems as iron is preferentially used for production of haemoglobin, and in iron deficiency, iron is sequestered for the production of hemoglobin and so non-heme tissues such as skeletal muscle, the heart and the brain will become iron deficient a long time before overt anaemia is obvious (Rao and Georgieff, 2002). Once born, infant brain iron levels decrease in the first 6 months of life, which roughly equates to the onset of myelination. The most sensitive period (and hence the period that can cause the most irreversible damage) is the period between 0 and 24 months of age. Iron deficiency in this period is correlated with poor auditory recognition memory, delayed cognitive development and poor response to external stimuli.
During pregnancy the mother sacrificially loads up the foetus with the result that many women can become iron deficient during pregnancy. Children born to mothers with low serum ferritin tend to have low serum ferritin as well, and that there is a positive correlation between maternal serum ferritin and the resultant iron reserves in the children (Gaspar etal, 1993; Jaime-Perez etal, 2005; Shao etal, 2012; Lee et al, 2016). Iron loading of the foetus occurs progressively during foetal development and depends upon gestational age, and also the iron levels of the mother, hence the shorter the pregnancy and the lower the iron in the mother, the lower the iron in the foetus.
From Siddappa etal, 2007
Iron deficiency is associated with lower activity of an important enzyme, aconitase and in the elderly low aconitase activity is associated with lower mini mental score estimation
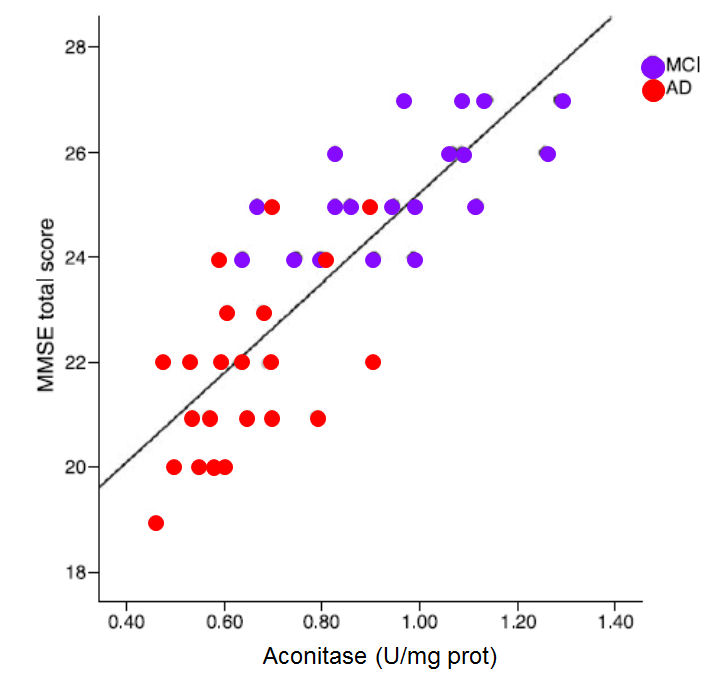
MMSE score against the activity of the enzyme aconitase (Figure. Data from Mangialasche etal,2015)
Reduced iron deficiency, particularly when coupled with vitamin B12 deficiency is associated with lower activity of the Krebs cycle enzyme, Aconitase, which in turn results in lower energy entering Krebs cycle, higher urinary citrate, and lower Mini Mental score. Lower aconitase activity has been found in the cerebellum and Brodman areas of the brains in those with autism.
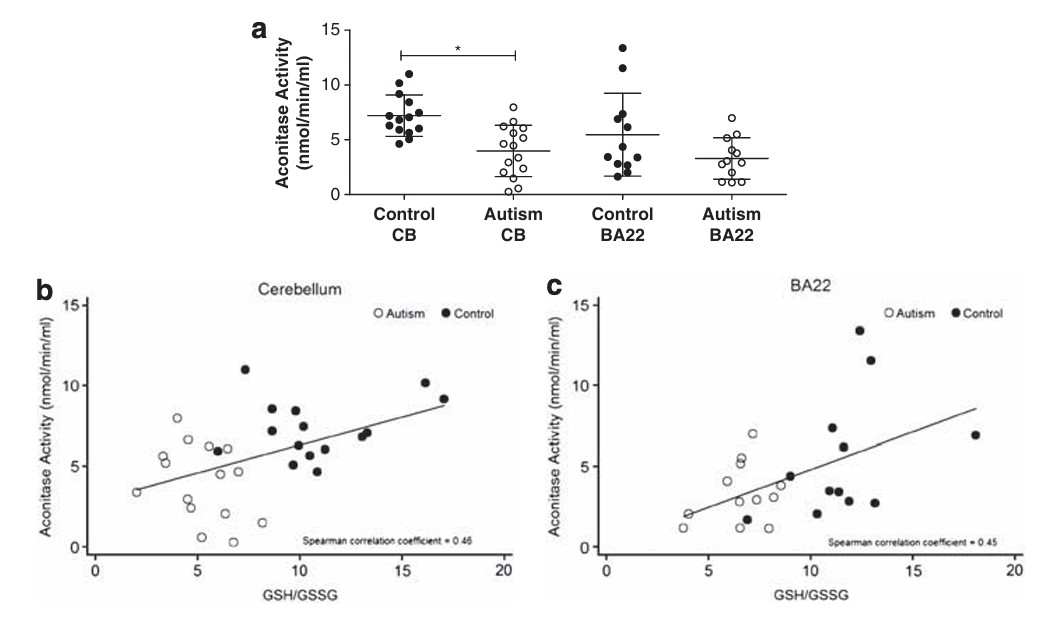
Reduced Aconitase activity in the cerebellum and Brodman area of the brain in control and those with autism (Fig from Rose etal, 2012)
Iron Deficiency in autism
Iron deficiency is the second most preventable cause of delayed development (Domellöf 2013; Berglund and Domellöf, 2014;Wiegersma etal, 2019), and globally 25% of children have Iron Deficiency (Domellöf 2013). It has been known for some time that the level of iron in the brains of autistic children is much lower than in normal individuals (Bener 2017; Latif etal, 2002,; Drosman etal, 2006, 2007, Heuguner etal, 2012; Reynolds etal, 2012; Youssef etal, 2013; Sidrak etal, 2014). Iron deficiency was associated with lower haemoglobin, haematocrit, and MCV values (Gunes 2017), with a negative correlation between lower haematocrit levels and degree of symptomatology (Sidra 2014). Iron deficiency in neonates has also been associated with poor emotional outcomes (Kim, 2014; Zumbrennen-Bullough 2004), recognition memory (Geng 2015), poor neural maturation (Armin 2010; Choudhury 2015; Armony-Sivan 2004). Iron is essential for learning and memory, and both the cholinergic and glutamatergic neurotransmission pathways are regulated by iron, and play a huge role in memory performance (Han 2015; Rodlowski and Johnson, 2013) and in the production of myelin by myelin-producing oligodendrocytes (Rosato-Siri 2017; Roth 2016).
Delayed Chord Clamping
It is recommended that in order to maximize iron levels in the new-born that clamping of the chord is delayed (Mercer etal, 2018).
Function of Iron in the body
For many years, it was thought that iron was solely involved as part of the structure of the heme molecule such as in the structure in hemoglobin. More recently it has been recognized as an important factor in the activity of iron sulphur proteins, such as aconitase, succinate dehydrogenase, and more recently gamma-aminobutyrate amino transferase (GABA-AT) and lipoate synthase. Iron is critical for myelination of nerve cells, and lack of myelination causes slower neuronal conduction, abnormal reflexes in children, and deficits in auditory and visual function (
Armin etal, 2010). Iron deficiency has also been associated with poorer language and global IQ, and social and attention problems. Iron-deficiency anemia is an advanced stage of iron deficiency, however, early Iron deficiency is associated with cognitive alterations in adolescents, and with developmental delay in children. Iron deficiency is generally associated with reduced levels of the iron storage protein, ferritin and ferritin levels are significantly lower in ASD kids (28.6 ug/L +/- 22 ug/L) when compared to normal individuals (152 ug/L +/- 142 ug/L). Deficiency of activity of Fe-S proteins has been shown to result in lower energy output in Krebs cycle and the electron transport chain, and also lack of emotional control due to lack of activity of GABA-AT, with increased anxiety. Increased levels of GABA are characteristic of iron deficiency (54),presumably due the lack of function of GABA-aminotransferase, an iron-sulphur protein, whose production is limited in iron and vitamin B12 deficiency.Iron has a critical role in the formation of the myelin sheath around neurons, and iron deficiency in mothers has shown a higher incidence of conditions such as ASD, and has also been associated with irreversible alterations in myelin (65). Iron deficiency in the brain precedes the signs of iron deficiency in RBC production. Serum ferritin levels below 76 ug/L are associated with abnormalities in neonatal recognition memory, and neuronal processing (66-71). Further, a recent study has shown that lower iron levels (as judged by serum ferritin) are associated with decreased brain activity and lower energy expenditure, as well as a reduced heart rate (31). Iron is known to be critical in neurodevelopment, and fetal iron deficiency has been shown to result in acute brain dysfunction, with long-lasting abnormalities even after repletion (72-77). Lower iron is also associated with restless leg syndrome and febrile seizures, which are common in children with ASD (Sherjil etal 2010; Fallah etal, 2014; Gillberg etal, 2017; McCue etal, 2016; Hara 2007). Seizure rates were increased in children on soy infant formula, presumably due to the lower iron content (81). Iron deficiency is associated with a greater risk of headache, particularly if blood pressure is elevated. Worryingly, iron deficiency is still be defined by clinicians in term of iron deficiency anemia, and values of 12 or 15 ug/L ferritin being commonly used to assign deficiency (84). Thus, children who have many of the signs assigned to iron deficiency, such as reduced cognition, developmental delay, depression, poor neuronal processing, anxiety, migraine headaches, restless leg syndrome and febrile seizures, are not being treated for iron deficiency due to clinicians sticking to archaic, anemia defined, definitions of iron sufficiency. Our studies have shown reduced energy production by aconitase and succinate dehydrogenase when serum ferritin levels drop below 70 ug/L (85-88). This reduced energy production can be seen by the massive increase in energy lost in the form of citrate as iron (as measured by ferritin) levels drop. Our studies have shown that as ferritin levels drop from 70 to 20 ug/L (seen not deemed as iron deficient by most pathology labs) up to 9 times the amount of citrate is lost to those who are iron replete. This means that effective energy consumption by the brain will also drop. Such energy loss, if extrapolated "planet-wise" is catastrophic environmentally. Despite these known benefits of diets replete in iron, 15-42% of children are born iron deficient (Bastian etal, 2020), due primarily to exceedingly poor dietary choices of the mothers.
Iron deficiency has been shown to cause iron deposition in white matter in the brain and in oligodendrocytes (mylein producing cells). The dysregulation of iron metabolism ultimately leads to neurological, behavioural and nociceptive impairments (100). Iron deficiency can lead to anemia, changes in cognitive performance, emotions, behavior, reduced exercise capacity, and myocardial functional and structural changes (Fava etal, 2019; Jankowaska et al 2011).
Determination of Iron Deficiency
One of THE biggest problems in determining iron deficiencies in the mothers revolves around the definition of iron deficiency. Generally Iron deficiency has not been related to biochemical iron deficiency, but rather to some arbitrary value correlating ferritin values with anaemia. These arbitrary values vary greatly depending upon the country in which the measurements are taken, and do not reflect various biochemical parameters whose values measure markers of iron deficiency. Thus, Iron deficiency as judged by haematological parameters occurs at around 15-20 ug/L ferritin in adults, however, metabolically iron deficiency can be observed when ferritin values drop below 70 ug/L and evidence of altered cell metabolism occurs when ferritin drops below 100 ug/L. Further, there appears to be a general ignorance in the medical profession of the role of iron in the body, and what effect lowered Haemoglobin and haematocrit have on oxygen carrying capacity of the blood, nor the massive reduction in energy seen in Krebs cycle as a result of decreased activity of the enzyme aconitase, when ferritin levels drop below 60 ug/L. This observation would support the contention of several workers that serum ferritin below 74 ug/L is indicative of abnormalities in neonatal recognition memory, in reflexes and in the myelin-dependent speed of neuronal processing (Georgieff, 2017, Geng etal, 2015; Armony-Sivanetal, 2004; Armin et al, 2010)
Definitions of iron deficiency also vary from country to country, with a cut-off for ferritin of 12 ug/L in Indonesia (Yusrawati etal, 2018), 12 ug/L in Brazil (de Sa etal, 2015). In Saskatoon, the range is 5 to 70 ug/L, which would mean that technically the vast population of this city is iron deficient.This is further complicated by the definitions associated with iron deficiency such as levels of haemoglobin and haematocrit, both of which are different from biochemical evidence of iron deficiency. Thus, iron deficiency can be categorized into three main types, iron deficiency with anaemia, which relates purely to the number of Red Blood Cells, biochemical iron deficiency without anaemia, in which biochemical parameters of iron deficiency can be measured, or iron sufficiency. Of these, iron sufficiency appears occur above 70 ug/L ferritin. In certain countries and even in different states in the US, Definition of iron sufficiency varies tremendously. Thus, in Texas Hb ranges are 10.2-12.7 g/dl, and so the average person in the state is biochemically iron deficient. NJ-USA 11.7-15.5 g/dl, AZ 12.0-16.9 g/dl. In the UK, in contrast the range is 12.0 - 15.0. Ranges for ferritin are equally variable, with NJ USA 16-154 ng/ml, Hong Kong 21.8-274 ng/ml.
A recent summary of guidelines for management of iron deficiency, world-wide, recommended serum ferritin values should be above 100 ug/L (Peyrin-Biroulet etal, 2015; Fava etal; 2019).
Hypotonia in Autism
Low iron levels as judged by low serum ferritin is associated with poor muscle tone. Low muscle-tone, hypotonia, is very common in autism, and early diagnosis of autism should be suspected in children with hypotonia, as early as "Hypotonia is a recognizable marker of ASD and may serve as a "red flag" to prompt earlier recognition and neurodevelopmental evaluation toward an autism diagnosis." (Gabis etal 2021; Lopez-Espejo, etal, 2021). Hypotonia is associated with decreased language development and IQ in autism (Osljeskova etal, 2007; Fillano etal, 2002). Hypotonia is the result of reduced energy transfer in Krebs cycle and due to inefficiency of the electron transport chain. The lower the iron, the lower the energy. The lower the ferritin, the greater the hypotonia. Hypotonia is also very common in Down Syndrome (Buterbaugh etal, 2013)
.Hypermobility in Autism
Cross-linking of collagen involves the iron-sulphur proteins, lysyl hydroxylase and prolyl hydroxylase, the functions of which are reduced in low serum ferritin concentrations (Link). Loss of cross-linking leads to hypermobility of joints, which is a common issue in Autism (Casanova et al, 2020; 2018; Baeza-Velasco etal, 2020; Tedla etal, 2021; Kindgren etal, 2021), and should also be a red flag for both low iron and autism.
Resolving Iron Deficiency in Autism
Iron deficiency is extremely common in ASD and this needs to be addressed if the child is going to have any chance of normal development. For neurotypically normal development large quantities of iron are required for the process of myelination of the brain and peripheral nervous system. Estimates are 7 mg/day for children 1 to 8 years old. This estimate is of bioavailable iron, NOT of iron in the supplement. It is best to introduce iron containing meats, such as beef or chicken liver, clams, mollusks or mussels or oysters >> beef, lamb goat, deer, bison, sardines turkey, all of which have much higher iron contents than chicken or pork. Non-meat sources of iron are extremely poorly absorbed, even despite this studies have shown that 6 mg/kg/24 h ferrous sulphate when given orally is able to restore normal nerve conduction velocities in children with iron deficiency (Kabakus etal, 2002). A recent study using oral iron-bisglycinate given at 3 mg/kg (90 mg/30 kg child) resulted in a modest increase in serum ferritin from 20 ug/L to 40 ug/L after 45 days (Name etal, 2018), supporting the observations that oral absorption of non-heme iron is very inefficient. One study suggested that there is actually more iron that is available from the rusting of an iron pot than is available from cooking non-heme foods. Studies on iron absorption from beans suggests that oral bioavailabiity of iron may be as little as 0.4% (Junqueira-Franco etal, 2018). Iron deficiency and Restless Leg Syndrome are also common in ADHD (Lopez etal, 2019)
Iron Deficiency and altered Thyroid Function
Iron is involved in the function of the enzyme thyroid peroxidase, and as levels of ferritin in serum drop, the activity of the enzyme becomes compromised and levels of thyroid hormone drop with an accompanying rise in TSH (Eftekhari etal, 2006), potentially making individuals with low iron hypothyroidic (Veltri et al, 2016' Li etal, 2016; Tenq etal, 2018), which would result in functional B2 deficiency (Maldonado-Araque etal, 2018). This effect would be exacerbated in pregnancy as levels of iron in the mother drop as they "feed" the foetus with iron. Such hypothyroxinemia induced by iron deficiency would further impair normal brain development (Hu etal, 2016). In this regard, "both perinatal hypothyroiximeia and perinatal iron deficiency are associated with poor neurodevelopment in offspring" (Hu et al 2016)Further, iron deficiency induced hypothyroidism would result in functional vitamin B2 deficiency, which then results in functional vitamin B12 deficiency.
Resolving Iron Deficiency in Pregnant mothers
Iron deficiency is extremely common in vegetarian adults, and amongst female vegetarians, 12-79%, depending upon the study, where found to have exceedingly low serum ferritin, <12 ug/L (Pawlak etal, 2016). Iron deficiency in becoming increasingly common in women of reproductive age, and a recent study identified iron deficiency to be common in Canada (Ave ferritin 36.9 ug/L) and Malaysia (Ave Ferritin 38.4 ug/L) (Aljaadi etal, 2019). Children of mothers with levels that low would be in grave danger of very low brain iron levels, and to potentially have irreversible brain damage. Studies carried out in Denmark by Milman and co-workers (2006) looked at iron deficiency in pregnant mothers and came up with the following suggestions: "80-100 mg ferrous iron/day to women having ferritin < or =30 microg/l and 40 mg ferrous iron/day to women having ferritin 31-70 mug/l. Iron deficiency in pregnancy is often associated with restless leg syndrome and/or pre-eclampsia (Ramirez etal, 2013; Minar etal, 2017), The lower the Hb level, the more common the RLS (Minar etal, 2017). According to Hunt (2003)"It is suggested to admin 40 mg ferrous iron/day to women having ferritin < or =70 microg/l. Women with ferritin >70 microg/l have no need for iron supplement." A recent study in the UK reported that 46% of women were anemic at some point during pregnancy (Benson etal, 2020). Despite this, the RACGP does not recommend iron supplements in pregnancy - UNBELIEVABLE. (Iron availability from food sources has vastly different absorption characteristics, thus heme-iron, such as that found in red meat, and seafood, is much more efficiently absorbed than similar quantities of iron found in vegetables, particularly those containing iron-complexing/chelating molecules such as phytates. The data below is from a study comparing the uptake of iron (Fe) from heme iron (open bars) to that from non-heme iron sources (closed bars) (Hunt, 2003). During pregnancy, pregnant women need 27 mg of absorbed iron per day.
During pregnancy, there is a marked drop in ferritin levels in the mother, as firstly her blood volume increases dramatically, and secondly, the foetus starts to accumulate iron particularly in the third trimester. For this reason determination of iron sufficiency is somewhat harder during pregnancy.
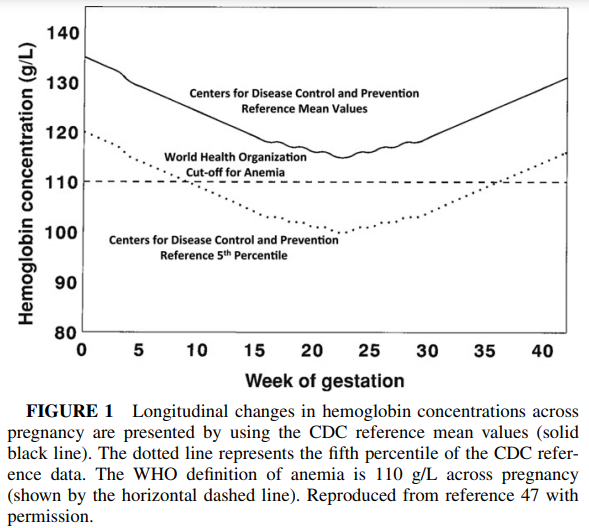
O'Brien and Ru, 2017

Besnon etal, 2021 Comparison of serum ferritin in supplemented (top line) and non-supplemented mothers (bottom line)
Iron deficiency anemia is very common in pregnant women with approximately 25% of women having iron deficiency anaemia during pregnancy. Despite this at present routine administration of iron supplements in pregnancy is not recommended in Australia, the UK, New Zealand and the US. This is almost incredulous, particularly considering that iron deficiency is the second most preventable cause of mental retardation in the world (Frayne and Pinchon, 2019}
Iron Supplementation in Pregnant mothers
Mothers should ensure iron sufficiency before they become pregnant with a minimum of 70 ug/L ferritin being desirable. Supplementation of functionally B2 or B12 deficiency with inorganic iron supplements such as Spatone having been found to be almost useless.
Iron Deficiency and Vitamin D deficiency
Iron is required in several steps in the synthesis of vitamin D, and iron deficiency has been associated with lower vitamin D levels (Sim etal, 2010; Blanco-Rojo etal, 2013; Lee, etal, 2018;Liu, etal, 2015; Constantini, etal 2010; Sharma, etal, 2015;
Malczewska-Lenczowska etal, 2018; Nur-Eke etal, 2020). Potentially iron deficiency leads to reduced activity of alpha-1-hydroxylase, which is involved in the production of 1, 25-dihydroxycalciferol, and also 25-hydroxylase, which is involved in the production of 25-hydroxyvitamin D. (calciferol), and is in line with the Nexus Theory™ of autism. Further, an association between iron deficiency and vitamin D deficiency has been found in children with autism (Bener etal, 2017; Kaymak etal, 2018; Uwaezuoke 2017)Associated Deficiencies in Autism
The majority of studies looking at iron deficiency in children and in autism have now addressed the likely co-deficiency of vitamin B12, however, one could assume that a diet low in iron would also be a diet low in vitamin B12. Every child that we have data for who has autism is also deficient in active vitamin B2 (FMN and FAD) and is deficient in active vitamin B12 (Adenosyl and Methyl B12), these deficiencies also have to be addressed or the child will potentially have a considerable developmental delay.
Energetics of Iron Deficiency in Autism
Iron has a major role in the conversion of energy generated from the metabolism of fats, sugar and protein, and as iron levels drop (as measured by serum ferritin), the energy conversion via the iron-sulpur enzyme aconitase drops rapidly and there is an increased excretion of citrate in urine - representing metabolic energy that cannot be processed. Iron deficiency in the diet is an environmental disaster, as it represents a highly inefficient use of consumed calories, with some children "wasting" upwards of 80% of consumed calories. The corollary to this is that the highly energy dependent neurons in the brain are effectively starved of energy as iron levels drop. This then results in impaired mitochondrial respiration, lower intracellular ATP production, and leads to chronic neuronal energy insufficiency (Raichle and Gusnard, 2002), with a subsequent reduction in mitochondrial speed with reduced hippocampal neuronal development (Bastian etal, 2019). Decreasing levels of ferritin in serum have been correlated with higher levels of "wasted" citrate in urine - Personal obvervations)
Relationship between serum ferritin (vertical axis) and urinary citrate (horizontal axis).
Hence, from an energetic point of view lower iron means:
-
Lower oxygen carrying capacity of blood due to lower Haemoglobin
-
Lower intracellular oxygen capacity due to lower activity of myoglobin
-
Lower activity of the iron-sulphur protein, aconitase leading to massive energy loss in the citric acid cycle.
-
Lower Electron Transport activity due to lower activity of iron-sulphur and heme proteins in the Electron Transport Chain.
Copyright.
The descriptions and findings on iron and autism, is the property of B12 Oils Pty Ltd. Reproduction in whole or in part constitutes an infringement in the Copyright law. Copyright infringement carries serious penalties.
References
-
Ferriera etal. Multilevel impacts of iron in the brain" The cross talk between neurophysiological mechanisms, cognition, and social behaviour. Pharmaceuticals 2018 12 https://www.ncbi.hlm.nih.gov/pmc/articles/PMC6789770/
-
Yusrawati etal Differences in brain-derived neurotrophic factor between neonates born to mothers with normal and low ferritin. Asia Pac J Clin Nutr 2018 27: 389-392
-
Schmidt et al Maternal intake of supplemental iron and risk of autism spectrum disorder 2014 PMID 25249546
-
Radlowski and Johnson Perinatal iron deficiency and neurocognitive development Front Hum Neurosci 2013 7, 585
-
Rao and Georgieff Perinatal aspects of iron metabolism https://www.ncbi.nim.nih.gov/pubmed/12477276
-
Amin etal In utero iron status and auditory neural maturation in premature infants as evaluated by auditory brainstem response. PMID 19939407
-
Domellöf M. (2013). Iron and other micronutrient deficiencies in low-birthweight infants. Nestle Nutrition Institute workshop series, 74, 197–206. https://doi.org/10.1159/000348772
-
Wiegersma, A. M., Dalman, C., Lee, B. K., Karlsson, H., & Gardner, R. M. (2019). Association of Prenatal Maternal Anemia With Neurodevelopmental Disorders. JAMA psychiatry, 76(12), 1294–1304. https://doi.org/10.1001/jamapsychiatry.2019.2309
-
Berglund, S., & Domellöf, M. (2014). Meeting iron needs for infants and children. Current opinion in clinical nutrition and metabolic care, 17(3), 267–272. https://doi.org/10.1097/MCO.0000000000000043
-
Hare etal, 2013 A delicate balance: Iron metabolism and diseases of the brain... PMIC 3715022
-
Kwik-Uribe etal, 2000 Chronic marginal iron intakes during early development in mice result in persistent changes in dopamine metabolism and myelin composition.PMID 11053527
-
Choudhury etal 2015 Latent iron deficiency at birth influences auditory neural maturation in late preterm and term infants PMID 26310540
-
Armony-Sivan etal 2004 Iron status and neurobehavioral development of premature infants PMID 15318248
-
Mercer JS, Erickson-Owens DA, Deoni SCL, Dean DC 3rd, Collins J, Parker AB, Wang M, Joelson S, Mercer EN, Padbury JF. Effects of Delayed Cord Clamping on 4-Month Ferritin Levels, Brain Myelin Content, and Neurodevelopment: A Randomized Controlled Trial. J Pediatr. 2018 Dec;203:266-272.e2. doi: 10.1016/j.jpeds.2018.06.006. Epub 2018 Jul 6. PMID: 30473033; PMCID: PMC6259583.
-
Wenger etal, 2017 Effect of iron deficiency on simultaneous measures of behavior, brain activity and energy.. PMID 28784049
-
Zumbrennen-Bullough etal 2014 Abnormal brain iron metabolism in Irp2 deficient mice is associated with mild neurological and behavioral impairments PMID 24896637
-
Georgieff 2008 The role of iron in neurodevelopment: fetal iron deficiency and the developing hippocampus PMID PMC2711433
-
Georgieff MK, Krebs NF, Cusick SE. The Benefits and Risks of Iron Supplementation in Pregnancy and Childhood. Annu Rev Nutr. 2019 Aug 21;39:121-146. doi: 10.1146/annurev-nutr-082018-124213. Epub 2019 May 15. PMID: 31091416; PMCID: PMC7173188.
-
Wang M. Iron Deficiency and Other Types of Anemia in Infants and Children. Am Fam Physician. 2016 Feb 15;93(4):270-8. PMID: 26926814.
-
Sherjil etal 2010 Iron deficiency anaemia - a risk factor for febrile seizures in children PMID 22338422
-
Fallah etal 2014 Iron deficiency and iron deficiency anemia in children with first attack of seizure and on healthy control group.... PMC PMC4135276
-
Gillberg etal 2017 Febrile seizures and epilepsy: Association with autism and other neurodevelopmental disorders in the child....... PMID 28754226
-
McCue etal 2016 Prevalence of non-febrile seizures in children with idiopathic autism spectrum disorder and their unaffected siblings....... PMID 27894273
-
Kabakus etal 2002 Reversal of iron deficiency anemia-induced peripheral neuropathy by iron treatment of children with iron deficiency anemia. PMID: 12200980
-
Siddappa etal 2007 The assessment of newborn iron stores atbirth: a review of teh literature and standards for ferritin concentrations
-
Mangialasche etal, 2015 Lymphocytic mitochondrial aconitase activity is reduced in Alzheimer's disease and mild cognitive impairment. PMID: 25322927
-
Pawlak etal, 2016. Iron status of Vegetarian Adults: A literature review.
Sage
-
Hara 2007 Autism and epilepsy: a retrospective follow-up study PMID 17321709
-
Fallah etal 2016 Evaluation efficacy of ferrous sulfate therapy on headaches of 5-15 years old iron deficient children with migraine PMC
-
Giulivi et al Mitochondrial dysfunction in autism. J. Am. Med Assoc 2010 304: 2387-2396
-
Chauhan etal Brain region-specific deficit in mitochondrial electron transport chain complexes in children with autism. J. Neurochem. 2011 117: 209-22
-
Tang et al Mitochondrial abnormalities in temporal lobe of autistic brain. Neurobiol Dis 2013 54: 349-361
-
Goh et al Mitochondrial dysfunction as a neurobiological subtype of Autism Spectrum Disorder.. JAMA psychiatry 2014 71: 665-671
-
Napoli et al Deficits in bioenergetics and impaired immune response in granulocytes from children with autism. Pediatrics 2014 133:e1405-10
-
Zumbrennen-Bullough; et al. Abnormal brain iron metabolism in Irp2 deficient mice is associated with mild neurological and behavioural impairment. PloS One 2014 e98072 PMC 4045679
-
Sherjil et al Iron deficiency anaemia - a risk factor for febrile seizures in children 2010 PMID 22338422
-
Westmark Soy infant formula and seizures in children with autism: a retrospective study. 2007 PMID 24622158
-
de Sa et al. Anemia in pregnancy: impact of weight and in the development of anemia in the newborn. Nutr Hosp 2015 32:2071-2079
-
Gaspar et al. Relationships between iron status in pregnant women and their newborn babies.. Acta Obstet Gynecol. Scand. 1993 72:5234-7
-
Jaime-Perez et al. Sub-optimal fetal iron acquisition under a maternal environment. Arch Med Res. 2005 36: 598-602
-
Shao et al. Maternal serum ferritin concentraion is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. J. Nutri. 2012 142:2004-9
-
Lee et al. Prevalence of anaemia and associations between neonatal iron status, hepcidin and maternal iron status among neonates born to pregnant adolescents. Ped Res. 2016 79:42-8
-
Kabakus et al, Reversal of iron deficiency anemia-induced peripheral neuropathy by iron treatment of children with iron deficiency anemia. J Trop Ped 2002 48: 204-9
-
https://nutritionovereasy.com/2015/05/cast-iron-pans-can-increase-your-iron-intake/
-
Brittin HC, Nossaman CE. Iron content of food cooked in iron utensils. J Am Diet Assoc. 1986;86:897–901
-
Junqueira-Franco etal, Iron absorption from beans with different contents of iron, evaluated by stable isotopes.
PMID
29779806
-
Eftekhari etal The relationship between iron status and thyroid hormone concentration in iron-deficient adolescent Iranian girls. https://www.ncbi.nlm.nih.gov/pubmed/16500878
-
Lopez, R., Micoulaud Franchi, J. A., Chenini, S., Gachet, M., Jaussent, I., & Dauvilliers, Y. (2019). Restless legs syndrome and iron deficiency in adults with attention-deficit/hyperactivity disorder. Sleep, 42(5), zsz027. https://doi.org/10.1093/sleep/zsz027
-
Veltri etal, 2016 Prevalence of thyroid autoimmunity and dysfunction in women with iron-deficiency in early pregnancy... https://www.ncbi.nlm. nih.gov/pubmed/27450694
-
Li etal, 2016 The relationship between iron deficiency and thyroid function in Chinese women during early pregnancy https://www.jstage.jst.go.jp/article/jnsv/62/6/62_392/_article
-
Tenq etal, 2018 Iron deficiency may predict greater risk for hypthyroxinemia: A retropective cohort study of pregnant women in China https://www.ncbi.nlm.nih.gov/pubmed/29968513
-
Maldonado-Araque etal, 2018 Iron deficiency is associated with hypothyroxinemia ... https://www.ncbi.nlm.nih.gov/pubmed/29700318
-
Yu et al Iron deficiency, an independent risk factor for isolated hypothyroxinemia in pregnant ...... https://www.ncbi.nlm.nih.gov/pubmed/25599388
-
Hu etal Perinatal iron deficiency-induced hypothyroxinemia impairs early brain development https://www.ncbi.nlm.nih.gov/pubmed/27231981
-
Name etal Iron Bisglycinate Chelate and Polymaltose Iron for the Treatment of Iron Deficiency Anemia: A Pilot Randomized Trial PMID 6416187
-
Milman et al. Body iron and individual iron prophylaxis in pregnancy - should the iron dose be adjusted according to serum ferritin. Ann Hematol. 2006 85: 567-73.
-
Gabis LV, Shaham M, Leon Attia O, Shefer S, Rosenan R, Gabis T, Daloya M. The Weak Link: Hypotonia in Infancy and Autism Early Identification. Front Neurol. 2021 Feb 4;12:612674. doi: 10.3389/fneur.2021.612674. PMID: 33613430; PMCID: PMC7891038
-
Lopez-Espejo MA, Nuńez AC, Moscoso OC, Escobar RG. Clinical characteristics of children affected by autism spectrum disorder with and without generalized hypotonia. Eur J Pediatr. 2021 Oct;180(10):3243-3246. doi: 10.1007/s00431-021-04038-7. Epub 2021 Apr 14. PMID: 33855616.
-
Oslejskova H, Dusek L, Makovska Z, Rektor I. Epilepsia, epileptiform abnormalities, non-right-handedness, hypotonia and severe decreased IQ are associated with language impairment in autism. Epileptic Disord. 2007 Dec;9 Suppl 1:S9-18. doi: 10.1684/epd.2007.0154. PMID: 18319196
-
Fillano JJ, Goldenthal MJ, Rhodes CH, Marín-García J. Mitochondrial dysfunction in patients with hypotonia, epilepsy, autism, and developmental delay: HEADD syndrome. J Child Neurol. 2002 Jun;17(6):435-9. doi: 10.1177/088307380201700607. PMID: 12174964.
-
Casanova EL, Baeza-Velasco C, Buchanan CB, Casanova MF. The Relationship between Autism and Ehlers-Danlos Syndromes/Hypermobility Spectrum Disorders. J Pers Med. 2020 Dec 1;10(4):260. doi: 10.3390/jpm10040260. PMID: 33271870; PMCID: PMC7711487.
-
Baeza-Velasco C, Cohen D, Hamonet C, Vlamynck E, Diaz L, Cravero C, Cappe E, Guinchat V. Autism, Joint Hypermobility-Related Disorders and Pain. Front Psychiatry. 2018 Dec 7;9:656. doi: 10.3389/fpsyt.2018.00656. PMID: 30581396; PMCID: PMC6292952.
-
Tedla JS, Asiri F, Alshahrani MS, Gular K. Hypermobility among children with autism spectrum disorders and its correlation with anthropometric characteristics. J Pak Med Assoc. 2021 Apr;71(4):1076-1080. doi: 10.47391/JPMA.436. PMID: 34125746.
-
Kindgren E, Quińones Perez A, Knez R. Prevalence of ADHD and Autism Spectrum Disorder in Children with Hypermobility Spectrum Disorders or Hypermobile Ehlers-Danlos Syndrome: A Retrospective Study. Neuropsychiatr Dis Treat. 2021 Feb 10;17:379-388. doi: 10.2147/NDT.S290494. PMID: 33603376; PMCID: PMC7882457.
-
Casanova EL, Sharp JL, Edelson SM, Kelly DP, Casanova MF. A Cohort Study Comparing Women with Autism Spectrum Disorder with and without Generalized Joint Hypermobility. Behav Sci (Basel). 2018 Mar 17;8(3):35. doi: 10.3390/bs8030035. PMID: 29562607; PMCID: PMC5867488.
-
Buterbaugh A, Mroczkowski HJ, Shankar SP, Visootsak J. Contribution of Family History in Co-occurring Down Syndrome and Ehlers-Danlos Syndrome. Ann Paediatr Rheumatol. 2013 Apr 1;2(4):165-167. doi: 10.5455/apr.081820131456. PMID: 24839582; PMCID: PMC4020180.
-
Aljaadi etal. Suboptimal biochemical riboflavin status is associated with lower hemoglobin and higher rates of anemia in a sample of Canadian and Malaysian women of reproductive age.J Nutrit, 2019
-
Ars etal 2019 Prenatal folate, homocysteine and vitamin B12 levels and child brain volumes. Br J. Nutr. 122: S1-S9.
-
Rose S, Melnyk S, Pavliv O, Bai S, Nick TG, Frye RE, James SJ. Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl Psychiatry. 2012 Jul 10;2(7):e134. doi: 10.1038/tp.2012.61. PMID: 22781167; PMCID: PMC3410618
-
Peyrin-Biroulet L, Williet N, Cacoub P. Guidelines on the diagnosis and treatment of iron deficiency across indications: a systematic review. Am J Clin Nutr. 2015 Dec;102(6):1585-94. doi: 10.3945/ajcn.114.103366. Epub 2015 Nov 11. PMID: 26561626.
-
Fava et al. Heart failure and iron deficiency 2019 https://www.ncbi.nlm.nih.gov/pubmed/30821294
-
Jankowska et al Iron deficiency predicts impaired exercise capacity in patients with cystolic chronic heart failure https://www.ncbi.nom.nih.gov/pubmed/22041326
-
https://www.thewomens.org.au/images/uploads/fact-sheets/Iron-Infusion-0718.pdf
-
Ramirez, J. O., Cabrera, S. A., Hidalgo, H., Cabrera, S. G., Linnebank, M., Bassetti, C. L., & Kallweit, U. (2013). Is preeclampsia associated with restless legs syndrome?. Sleep medicine, 14(9), 894–896. https://doi.org/10.1016/j.sleep.2013.03.013
-
Minár, M., Košutzká, Z., Habánová, H., Rusňák, I., Planck, K., & Valkovič, P. (2015). Restless legs syndrome in pregnancy is connected with iron deficiency. Sleep medicine, 16(5), 589–592. https://doi.org/10.1016/j.sleep.2014.11.023
-
H
unt, JR. 2003 Bioavailability of iron, zinc, and other trace minerals from
vegetarian diets. Am J Clin Nut, 78: 633S-9
-
Sim, J.J.; Lac, P.T.; Liu, I.L.A.; Meguerditchian, S.O.; Kumar, V.A.; Kujubu, D.A.; Rasgon, S.A. Vitamin D deficiency and anemia: A cross-sectional study. Ann. Hematol. 2010, 89, 447–452.
-
Blanco-Rojo, R.; Pérez-Granados, A.M.; Toxqui, L.; Zazo, P.; de la Piedra, C.; Vaquero, M.P. Relationship between vitamin D deficiency, bone remodelling and iron status in iron-deficient young women consuming
an iron-fortified food. Eur. J. Nutr. 2013, 52, 695–703. -
Lee, J.A.; Hwang, J.S.; Hwang, I.T.; Kim, D.H.; Seo, J.-H.; Lim, J.S. Low vitamin D levels are associated with both iron deficiency and anemia in children and adolescents. Pediatr. Hematol. Oncol. 2015, 32, 99–108.
-
Liu, T.; Zhong, S.; Liu, L.; Liu, S.; Li, X.; Zhou, T.; Zhang, J. Vitamin D deficiency and the risk of anemia: A meta-analysis of observational studies. Ren. Fail. 2015, 37, 929–934.
-
Constantini, N.W.; Arieli, R.; Chodick, G.; Dubnov-Raz, G. High prevalence of vitamin D insufficiency in athletes and dancers. Clin. J. Sport Med. 2010, 20, 368–371.
-
Sharma, S.; Jain, R.; Dabla, P.K. The role of 25-hydroxy vitamin D deficiency in iron deficient children of North India. Indian J. Clin. Biochem. 2015, 30, 313–317.
-
Bener A, Khattab AO, Bhugra D, Hoffmann GF. Iron and vitamin D levels among autism spectrum disorders children.Ann Afr Med. 2017 Oct-Dec;16(4):186-191. doi: 10.4103/aam.aam_17_17.
-
Kaymak Cihan M, Ünver Korğalı E. Is there an association between vitamin D level and iron deficiency in children?Arch Argent Pediatr. 2018 Dec 1;116(6):e736-e743. doi: 10.5546/aap.2018.eng.e736.
PMID: 30457722 -
Malczewska-Lenczowska J, Sitkowski D, Surała O, Orysiak J, Szczepańska B, Witek K.The Association between Iron and Vitamin D Status in Female Elite Athletes.Nutrients. 2018 Jan 31;10(2):167. doi: 10.3390/nu10020167.
PMID: 29385099 -
Nur-Eke R, Özen M.The Relationship between Vitamin D Levels and Iron Deficiency and Anemia in Adults Applied for Periodic Medical Examination.
Clin Lab. 2020 Jun 1;66(6). doi: 10.7754/Clin.Lab.2019.190918. -
Uwaezuoke SN. Vitamin D deficiency and anemia risk in children: a review of emerging evidence.Pediatric Health Med Ther. 2017 May 10;8:47-55. doi: 10.2147/PHMT.S129362. eCollection 2017.
-
Raichle, M. E., & Gusnard, D. A. (2002). Appraising the brain's energy budget. Proceedings of the National Academy of Sciences of the United States of America, 99(16), 10237–10239. https://doi.org/10.1073/pnas.172399499
-
Bastian, T. W., von Hohenberg, W. C., Georgieff, M. K., & Lanier, L. M. (2019). Chronic Energy Depletion due to Iron Deficiency Impairs Dendritic Mitochondrial Motility during Hippocampal Neuron Development. The Journal of neuroscience : the official journal of the Society for Neuroscience, 39(5), 802–813. https://doi.org/10.1523/JNEUROSCI.1504-18.2018
-
Bastian, T. W., Rao, R., Tran, P. V., & Georgieff, M. K. (2020). The Effects of Early-Life Iron Deficiency on Brain Energy Metabolism. Neuroscience insights, 15, 2633105520935104. https://doi.org/10.1177/2633105520935104
-
Benson, C. S., Shah, A., Frise, M. C., & Frise, C. J. (2021). Iron deficiency anaemia in pregnancy: A contemporary review. Obstetric medicine, 14(2), 67–76. https://doi.org/10.1177/1753495X20932426
-
Frayne J, Pinchon D. Anaemia in pregnancy. Aust J Gen Pract. 2019 Mar;48(3):125-129. doi: 10.31128/AJGP-08-18-4664. PMID: 31256475.
http://www.ncbi.nlm.nih.gov/pubmed/25599388
http://www.ncbi.nlm.nih.gov/pubmed/25150119
http://www.ncbi.nlm.nih.gov/pubmed/12487769
http://www.ncbi.nlm.nih.gov/pubmed/12097675
Copyright © 2018 B12 Oils. All Rights Reserved.
Reproduction in whole or in part in any form or medium without express written
permission is prohibited