
Vitamin B12 Deficiency and Reduced Methylation
-
There are over 200 enzymes in the body involved in methylation
-
The major methyl donor for methylation is S-Adenosyl-Methionine (SAM).
-
Maintenance of methylation is critically dependent upon MethylCo(III)B12 and the enzyme methionine synthase (MTR)
-
The methylation cycle is also dependent upon two enzymes that require functional B2 sufficiency, MTHFR and MTRR
-
Maintenance of functional vitamin B12 requires functional B2 deficiency,
-
Lack of functional B2 in leads to functional B12 deficiency and a reduction in methylation activities
-
Functional Vitamin B2 deficiency can lead to functional B6 deficiency, exacerbating functional B12 deficiency
-
Functional Vitamin B2 deficiency and associated functional B12 deficiency, can lead to functional folate deficiency.
-
Reduced methylation has been associated with many conditions including dementia, Parkinson's disease, Autism, Chronic Fatigue Syndrome.
-
Methylation deficiency results in lower production of creatine, leading to problems with cognitive function, learning, memory, attention, speech and language.
Vitamin B12 and the Methylation cycle
Vitamin B12, or more specifically methylcobalamin (also known as Methyl B12 or more accurately MethylCo(III)B12) is an essential co-factor (helper) in maintaining the activity of the methylation cycle. There are approximately 200 methylation reactions in the body that depend upon S-Adenosylmethionine (SAM) as the methyl donor for these methylation reactions. Methylation using SAM as a cofactor is the second most important use of a cofactor after ATP (Ducker and Rabinowitz, 2017). Transfer of energy from ATP generated at the electron transport chain, occurs by the addition of Phosphate to Creatine, to create creatine phosphate. Formation of creatine, though is also dependent upon methylation, and so the use of ATP technically involves methylation.
Maintenance of sufficient SAM requires repeated regeneration of methionine from homocysteine, by the MethylCo(III)B12-dependent enzyme Methionine Synthase (MTR).
Homocysteine + MethylCo(III)B12-[MTR] => methionine + Co(I)B12-MTR.
In order to regenerate MethylCo(III)B12, from Co(I)B12 the enzyme MTR is able to use 5-Methyl-THF as the "methyl" donor, and so initiate the "back-reaction"
Co(I)B12-[MTR] + 5MTHF => MethylCo(III)B12-[MTR] + THF
The tetrahydrofolate (THF), so generated then proceeds into the folate cycle where it is used in a variety of reactions including the synthesis of purines, the conversion of Deoxyuridine to thymidine, or to the regeneration of 5MTHF for use in the methylation cycle.
The first step in the methylation pathway arguably starts with the reaction of serine with THF to form 5,10-methylene-THF plus glycine using the vitamin B6 dependent enzyme, Serine-hydroxymethyltransferase (SHMT). In functional vitamin B2 deficiency, due to Iodine or Selenium deficiency (see B2), there can be a deficiency in the active form of vitamin B6 - PLP, and so the initiation of methylation is reduced. It will also be reduced in dietary folate deficiency!
THF + Serine-[SHMT-PLP] => 5,10-methylene-THF + Gly-[SHMT-PLP]. (NB PLP is often referred to as Pyridoxal-5-Phosphate - P5P).
Note, that the sudden activation of SHMT by restoring active B2 (as FMN), which then can activate dietary vitamin B6, can greatly amplify methylation and so rapidly increase many methylation reactions, such as the production of adrenalin.
At this stage 5,10-methylene-THF is stuck or trapped within the folate cycle, and must be processed by the FAD/NADP-dependent enzyme Methylene Tetrahydrofolate reductase (MTHFR), which converts 5,10-methylene-THF to 5-methyl-THF (5MTHF). This reaction can be reduced or blocked in functional B2 deficiency, and can be greatly reduced by certain mutations in the gene MTHFR, leading to the formation of the MTHFR protein with much lower reaction rates.
5,10-methylene-THF-[MTHFR-FAD/NADPH] => 5MTHF- [MTHFR-FAD/NAD]
Methionine, either produced by methylation of homocysteine, or from dietary intake is then used as an acceptor of Adenosine to make the universal Methylation donor, S-Adenosylmethionine (SAM), which then is involved in over 200 methylation reactions in the body, including the methylation of DNA, histones, myelinbasic protein, lysine, and in the production of creatine, adrenalin, CoQ10 and melatonin.
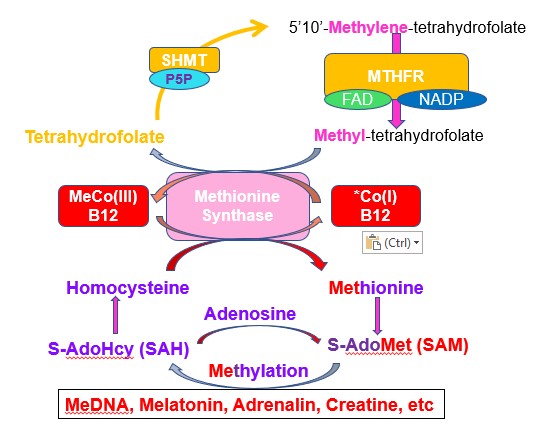
If methylCo(III)B12 is deficient, the methylation cycle is slowed and there is an increase in the level of homocysteine, and a increased ratio of SAH:SAM. There is also a reduced production of melatonin and more importantly CoQ10 and creatine, leading to continual reduced energy output, with resultant fatigue (CFS) and developmental delay in children.
What is not appreciated is that in order to process the homocysteine generated from the daily requirement of methionine (1.49 gm, 10 mMoles), the methionine synthase enzyme, which has around 1.36 ug (1nMole), methylCo(III)B12 (the daily RDA), the enzyme must cycle around 10 million times. During this time, the generation of 5,10-methylene THF and subsequent processing by MTHFR must happen 10,000 times. Hence the concept that adding additional 5MTHF to speed up methylation is very naive. The corollary to this is that it should not matter if you add folate, folinic acid, or 5MTHF, as they will all be converted to THF, and from there the SHMT enzyme will start off methylation. Cycling, though is dependent upon sufficient active B6 (P5P) and active B2 (FAD).
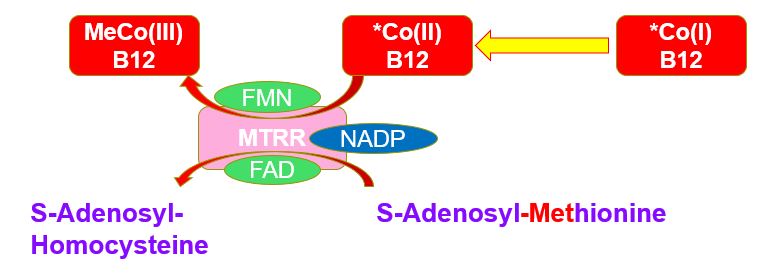
MTRR and oxidation of Co(II)B12
In
the reaction Homocysteine + MethylCo(III)B12-[MTR] => methionine + Co(I)B12-MTR, if there is no incoming 5MTHF for the back-reaction, the Co(I)B12-MTR is rapidly oxidized to Co(II)B12 which cannot partake in methylation. The Co(II)B12 can be "rescued" by the enzyme methionine synthase reductase (MTRR) in combination with SAM. The enzyme requires both FMN and FAD as well as NADPH.{MTRR-FMN/FAD/NADPH}Co(II)B12-MTR + SAM => {MTRR-FMN/FAD/NAD}Co(III)B12-MTR + SAH
Hence in functional vitamin B2 deficiency, there is both a decrease in the amount of 5MTHF that is produced by MTHFR, and as well there is a decreased activity of the enzyme MTRR, with the result that the amount of oxidized Co(II)B12 increases. This is subsequently released from MTR and MTRR and excreted from the cell, where it is rapidly bound by Haptocorrin, thereby contributing to an increase in circulating inactive Co(II)B12-Haptocorin, and contributes to elevated serum B12 levels seen in Paradoxical B12 deficiency (see https://b12oils.com/paradoxical.htm ). Of note, the activity of methionine synthase is critically dependent upon the presence of active MTRR, as lack of MTRR per se, or the presence of inactive MTRR results in the rapid inactivation of MTR (Yamada et al, 2006).
Dietary deficiency of riboflavin, Iodine, Selenium and/or Molybdenum and Methylation
Activation of dietary or supplemental vitamin B2 (riboflavin) requires a series of activation steps involving Thyroid Stimulating Hormone (TSH), thyroid hormone (T4), deiodinated T4 - triiodothyronine (T3), activation of riboflavin to FMN and finally modification of FMN to form FAD. Hence dietary insufficiency of any of Iodine, Selenium, Molybdenum or Riboflavin will lead to reduced activity of two of the major enzymes involved in maintenance of Methyl Co(III)B12 activity, MTHFR and MTRR. Reduction in MTHFR activity due to functional vitamin B2 deficiency, causes 5,10-methylene-THF to be stuck or trapped within the folate cycle, and there is an increase in 5,10-methylene-THF and a reduction in 5MTHF (Nijoult etal, 2004). Deficiency in the activity of MTHFR, MTR, and MTRR in the neonate leads to poor feeding, failure to thrive, vomiting, developmental delay, cerebral atrophy, hypotonia, ataxia, nystagmus, neonatal seizures and can cause visual disturbances (Hoffmann and Kolker, 2013). Knock-out of MTHFR alone results in defects in brain development (Ducker and Robinowitz, 2017).
Vitamin B2 deficiency and vitamin B6 deficiency
The activation of vitamin B6 is dependent upon FMN, one of the active forms of vitamin B2. FMN acts as a cofactor for the enzyme pyridoxine 5'-phosphate oxidase, which is involved in the conversion of pyridoxine to Pyridoxamine-5'-phosphate, the active form of vitamin B6. Deficiency of riboflavin, Iodine and/or Selenium will thereby affect the ability to activate vitamin B6 (Jungert etal, 2020). This in turn will lead to reduced activity of Serine Hydroxy Methyl Transferase (SHMT), and then a reduced production of 5,10-methylene-THF, with resultant drop in formation of 5MTHF and reduced methylation.
Elevated Homocysteine
Functional MethylCo(III)B12 deficiency is commonly associated with elevated homocysteine, as the rescue reaction using MTR is reduced
Homocysteine + MethylCo(III)B12-MTR => methionine + Co(I)B12-MTR.
Elevated homocysteine has been associated with many conditions including neural tube defects, Impaired childhood cognition (autism), macular degeneration, stroke, depression and anxiety (Chung etal, 2017) and cognitive impairment in the elderly. The association though may not be causative, but rather reflect potential deficiencies in active B2 as well as a reduction in over 200 methylation reactions in the body.
Homocysteine sits at a fork in the methylation cycle. One fork goes to the regeneration of methionine via methionine synthase, and is dependent upon Methyl Co(III)B12, whilst the other fork goes to the sulphation cycle via the enzyme cystathione beta synthase (CBS), and cystathione gamma lyase (CGL), both of which are dependent upon heme iron and the active form of vitamin B6, P5P. Hence deficiency in the active form of B2, FMN, will lead to reduced production of P5P and hence lead to reduced activity of CBS and CGL, as well as reduced regeneration of Methyl Co(III)B12 and so lead to elevated homocysteine.
Genetics and Methylation
Mutations in genes coding for SHMT, MTHFR, MTRR, CBS, and MTR can all affect or reduce the rate of methylation. The effect on genetics is much higher in conditions of vitamin B6 deficiency (CBS, SHMT), vitamin B2 deficiency (MTHFR, MTRR) and vitamin B12 (MTR).
The importance of the folate and methylation cycles for Methylation
It is essential that the both the folate and methylation cycles are working optimally in order to satisfy daily methylation requirements. Hence to process the 1.5 gm of methionine per day that enters the methylation cycle (around 10mmoles) you would need to make 10 mmoles of methyl B12, to donate the methyl group to homocysteine, and regenerate methionine. However, the RDA for methylcobalamin is only around 1.34 ug/day or 1 nmol/day, so you need to cycle B12 10,000,000 times per day, this then would need the same amount of folate, but the RDA is only around 441 ug/day or 1 umol, so folate has to cycle 10,000 times per day, just to process the 1.5 gm of methionine once. Now if all of the methyl groups on methionine only went to make the bodys' creatine usage (1.3 gm per day), this might be sufficient, BUT, you use 2-3 times this amount of creatine, and production of creatine is only about 40% of all methylation, so you have to cycle methionine at least 5-10 times, so there has to be an extensive cycling of both folate and B12.This is assuming optimal function of SHMT (vitamin B6 dependent), MTHFR (vitamin B2/B3 dependent) and MTRR (vitamin B2/B3 dependent).
Memory, Learning and Methylation
Many studies have shown the importance of histone methylation, specifically H3K4- methylation, in learning and memory (Collins etal, 2019; Gupta et al, 2010; Parkel etal, 2013; Poon etal, 2020; Kennedy etal, 2016; Morris etal, Pirisoto etal, 2016), and that reduced methylation, such as is seen in autism (due to functional B2/B12 deficiency), and in dementia is associated with intellectual disability (Parkel etal, 2013). Methylation deficiency results in lower production of creatine, leading to problems with cognitive function, learning, memory, attention, speech and language (Kondo et al, 2011z; Martine et al, 2009; Stork and Renshaw, 2005; Wood et al, 2009; Yildiz-Yesiloglu and Ankerst, 2006; Young et al, 2009),
Methylation and Sleep Disorders
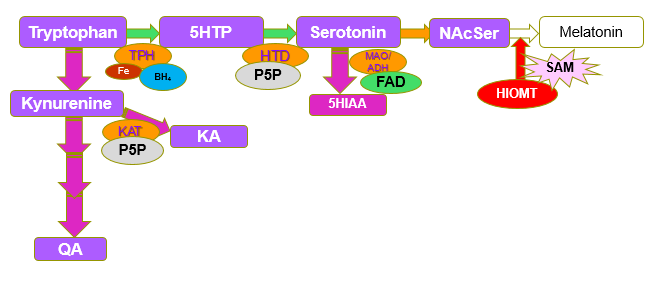
Synthesis of Melatonin from N-ActeylSerotonin by the enzyme Hydroxyindole-O-methyl Transferase (HIOMT). N-AcetylSerotonin-[HIOMT] + SAM => Melatonin=[HIOMT] + SAH. (Gallardo and Tamezzani 1975; Klein and Lines, 1969; Urry etal, 1972; Quay 1965;Kuwano and Takahashi, 1980; Yokim and Wallen 1975; )Deficiency in SAM leads to conditions such as poor sleep, poor maturation of the gut wall, and developmental delay due to lack of activation of neuronal stem cells and subsequent differentiation into myelin-producing oligodendrocytes in the brain. Sleep disorders are very common in those with functional B12 deficiency and are particularly prevalent in conditions such as autism (53-80%, Ballester etal, 2020) and dementia (Benca and Teodorescu., 2019'; Cipriani etal, 2015; Shenker and Sing, 2017). Despite the obvious role of methylation in the formation of melatonin, and its role in promotion of sleep, few researchers seem to understand this.

Melatonin and analogs that bind to the melatonin receptors
are important because of their role in the management of depression, insomnia,
epilepsy, Alzheimer’s disease (AD), diabetes, obesity, alopecia, migraine,
cancer, and immune and cardiac disorders.
Maturation of the cells that line the Gastrointestinal Tract requires the action
of Melatonin. Lack of melatonin production has been associated with reduced
uptake of calcium from the gut (Carpentieri et
al, 2014), as well as increased incidence of ulcerative colitis (Necefli
etal, 2006; Tasdemir etl, 2013), and reduced production of a
number of saccharidases including lactase, sucrase, glucoamylase,
isomaltase, and maltase (Trotta etal, 2021; Li etal, 2017). Poor gut health is a
feature of conditions such as ASD. Deficiency of Melatonin due to its role in
maturation of the gut mucosa leads to IBS-like symptoms, and sensitivity to
histamine, and can lead to MCAS.
Deficiency of melatonin also results in reduced expression of the Divalent Metal
Ion transporter, with reduction in uptake of ion such as calcium, magnesium,
zinc, manganese and Strontium. Deficiencies of which are characteristic of both
Autism and CFS. See
https://b12oils.com/melatonin.htm
Lack of methylation, leads to reduced synthesis of Melatonin. This
then results in over-production of serotonin, which leads to symptoms such as
depression, due to down-regulation of serotonin receptors. Subsequent treatment
with SSRIs is common, however it is wrong. The treatment should be the
resolution of the functional B12 deficiency,
Lack of methylation, leads to reduced synthesis of adrenalin. This
then results in over-production of dopamine and nor-adrenalin. Over-production
of dopamine leads to the anxiety, which is common in conditions associated with
vitamin B12 deficiency, such as autism, CFS, and dementia (Richdale etal, 2023).
Maturation of neuronal stem cells requires the combined action
of Melatonin and vitamin D. Lack of myelination has been associated with poor
myelination in the brain, and developmental and mental delay in conditions such
as ASD and in mental deterioration such as in dementia. Delayed myelination of
Broca's area in the brain is associated with lack of development of articulated
speech, a common feature of the Autism Spectrum Disorders (ASD). Potential modelling
of reduced activity of MTR/MTRR activity in reducing levels of active B2.
Methylation, melatonin and Gut Disorders
Methylation and Depression
Methylation and Anxiety
Methylation, melatonin and
Myelination in the brain
Increase in homocysteine and
reduction in Methylation with reducing levels of active B2.
Common Methylation reactions
Methylation is the second most common reaction in the body, second only to ATP production.
Synthesis of creatine by Guanidinoacetate-N-Methyl transferase (GNMT, GAMT) S-Adenosyl-L-methionine + Guanidinoacetate - [GAMT] => SAH + Creatine. Creatine is involved in the transfer of ATP from within the mitochondria into the cell. Lack of activity of the enzyme GAMT has been shown to give rise to many of the symptoms of autism. In addition lack of activity of GAMT leads to prolonged fatigue, similar to that in Chronic Fatigue Syndrome. Lack of activity of GNMT enzyme is associated with many symptoms associated with Autism and Alzheimer's Disease. In children GAMT deficiency can cause mental retardation, speech delay, seizures, behavioral changes, and movement disorders, including Muscular hypotonia, mild spasticity, and coordination disturbances (Longo etal, 2011; Pacheva etal, 2016; Stöckler et al, 1994; Mercimek-Mahmutoglu et al, 2006; Stockler-Ipsiroglu et al, 2014; Mercimek-Mahmutoglu et al 2014; O'Rourke et al, 2009; Araújo et al, 2005; Lion-François et al, 2006; Mercimek-Mahmutoglu et al, 2009; Leuzzi et al, 2013 Schulze et al, 2006;Verbruggen et al 2007; Morris et al, 2007; Item etal, 2004). Lower levels of vitamin B12 have been found in the brains of children with autism, suggesting a strong causal relationship (Zhang etal, 2016). Supplementation of creatine + Guanidinoacetate was found to be superior for muscle performance than creatine alone, possibly reflecting better transport of guanidinoacetate than creatine. Subjects were, however, functionally sufficient in vitamin B12, the same effect would not be expected in those who are methylation deficient (Semeredi, 2019). Studies on brain supplementation have shown better activity with GAA, BUT, this was also in B12 replete individuals (Ostojic etal, 2016; 2017; 2018). Further it does not seem possible to load either muscle or brain with GAA (Ostojic etal, 2018)
Synthesis of Adrenalin from Nor-Adrenalin by the enzyme Phenylethanolamine-N-Methyl Transferase (PNMT). Noradrenalin-[PNMT] + SAM => Adrenalin-[PNMT] + SAH. Deficiency in SAM leads to adrenal fatigue
Inactivation of histamine by the enzyme Histamine-N-Methyl Transferase. Histamine-[HNMT] + SAM => N-methylhistamine + SAH. Deficiency in SAM leads to histamine intolerance and in extreme cases can lead to a condition mistakenly named Mast Cell Activation Syndrome.
Synthesis of Phospatidylcholine (PC) from phosphatidylethanolamine (PEA) by the enzyme phosphatidylethanolamine N-methyltransferase. Phosphatidylcholine is a precursor to the synthesis of choline, for production of acetylcholine (Vance et al, 1997)- Reduced Acetylcholine production is common in Alzheimer's disease and autism (Ferri etal, 2005; Stanciu etal, 2019; Grossberg, 2017; Perry 1988; Dumas and Newhouse, 2011; Bartus etal, 1982). Conversion of PC to PEA has been proposed as being one of the biggest users of SAM in the body (Ducker and Rabonowitz, 2017).
Aspartate-N-methyl transferase (DDNMT), which produces NMDA from D-Aspartate. NMDA is involved in memory and learning. Lack of methylation potentially would cause a reduced ability for memory and learning and early signs of dementia.
Methylation of catecholamines using Catecholamine-O-Methyltransferase (COMT). Inactivation of catecholamine neurotransmitters. COMT is also involved in the inactivation of estrogen, and steroid hormones. Reduced COMT activity has been associated with a range of conditions, including anxiety (Desbonnet etal, 2012), preeclampsia (Pertegal et al, 2016), and several cancers (Matos etal, 2016).
Formation of Carnitine, following trimethylation of lysine with SAM and N-Lysine-methyltransferase. This is the precursor for the synthesis of carnitine, an essential carrier for transport of long chain fatty acids into mitochondria.
Numerous other enzymes see
N-methyltransferase - WikipediaReferences
Panth etal, 2019 A review of Iodine status of women of reproductive age in the USA. Biol Trace Element Res 188 208-220
Ducker GS, Rabinowitz JD. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017 Jan 10;25(1):27-42. doi: 10.1016/j.cmet.2016.08.009. Epub 2016 Sep 15. PMID: 27641100; PMCID: PMC5353360.
Morreale etal. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract. Res. Clin. Endocrinol. Metab. 2004, 18, 225–248.
Williams, G.R. Neurodevelopmental and neurophysiological actions of thyroid hormones. J. Neuroendocrinol. 2008, 20, 784–794.
Morreale de Escobar et al.. Iodine deficiency and brain development in the first half of pregnancy. Public Health Nutr. 2007, 10, 1554–1570.
Morreale de Escobar et al Role of thyroid hormone during early brain development. Eur. J. Endocrinol. 2004, 151, U25–U37.
Pop, et al Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin. Endocrinol. 1999, 50, 149–155.
Nijhout HF, Reed MC, Budu P, Ulrich CM. A mathematical model of the folate
cycle: new insights into folate homeostasis. J Biol Chem. 2004 Dec
31;279(53):55008-16. doi: 10.1074/jbc.M410818200. Epub 2004 Oct 20. PMID:
15496403.
Yamada et al, 2006 Human methionine synthase reductase is a molecular chaperone for human methionine synthase. PNAS 103, 25, 9476-9481
Hoffmann GF, Kolker, S. 2013 Deficiencies of methionine synthase reductase (cobalamin E defect) and methionine synthase (cobalamin G defect). Pediatric Neurology Part III in Handbook of Clinical Neurology 2013
Pop, et al. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3 year follow-up study. Clin. Endocrinol. 2003, 59, 282–288.
Kooistra, et al. Neonatal effects of maternal hypothyroxinemia during early pregnancy. Pediatrics 2006, 117, 161–167.
Zoeller, R.T.; Rovet, J. Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J. Neuroendocrinol. 2004, 16, 809–818.
Skeaff, et al Iodine deficiency does exist but is difficult to assess in individuals. N. Z. Med. J. 2009, 122, 101–102.
Costeira etal. Parameters of thryoid function throughout and after pregnancy in an iodine deficient population. Thyroid 2010, 20, 995–1001.
Chen et al, Cretinism revisited. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 39–50.
Rajatanavin et al. Endemic cretinism in Thailand: a multidiciplinary study. Eur. J. Endocrinol. 1997, 137, 349–355.
Zimmermann, M.B. Iodine deficiency. Endocr. Rev. 2009, 30, 376–408. 1
Pharoah, et al. Neurological damage to the fetus resulting from severe iodine deficiency during pregnancy. Lancet 1971, 297, 308–310.
Wolfe etal, 2011 Short-chain Acyl-CoA dehydrogenase deficiency. https://www.ncbi.nlm.nih.gov/pubmed/21938826
Mangels et al. The dietician's guide to vegetarian diets.
Leung etal Iodine status and thyroid function of Boston area vegetarians and vegans. J. Clin Endocrinol Metab. 2011 96
Jungert etal. Riboflavin as an important determinant of Vitamin B6 status in healthy Adults. J. Nutrition 2020 2699-2706
Ames etal High-dose vitamin therapy stimulates variant enzymes with decreased coenzyme binding affinity (increased K(m)): relevance to genetic disease and polymorphisms 11916749
Zimmerman and Kohrle The impact of iron and selenium deficiencies on iodine and thryoid metabolism: biochemistry and relevance to public health; https://www.ncbi.nlm.nih.gov/pubmed/12487769
Flechas 2020 Iodine deficiency and mental retardation https://www.youtube.com/watch?v=kZ-iDbgCupU&app=desktop
Chung etal Associations between serum homocysteine levels and anxiety and depression among children and adolescents in Taiwan. Scientific Reports 2017, 7 : 8330
Collins et al Histone H3 Lysine K4 methylation and its role in learning and memory. Epigen Chromatin. 2019, 12 :7
Gupta etal Histone methjylation regulates memory function. J Neurosci. 1020 30:3589-99
Parkel etal Histone H3 lysine methylation in cognition and intellectual disability disorders Learn Mem, 2013 20 : 570-9
Collins etal. Broad domains of histone 3 lysine 4 trimethylation are associated with transcription al activation in CA1 neurons of the hippocampus during memory formation. Neurobiol Learn Mem 2019 161: 149-57
Poon etal Memory and neuromodulation: a perspective of DNA methylation. Neruosci Biobehav Rev 2020 111: 57-68
Kennedy etal. TcF4 regulates synaptic plasticity, DNA methylation and memory function. Cell Rep. 2016 16:2666-2685
Ballester etal Sleep in autism: A biomolecular approach to aetiology and treatment. Sleep Med Rev. 2020 54:101357
Benca, RM and Teodorescu, M. Sleep physiology and disorders in aging and dementia. Handb Clin Neurol, 2019 167:477-493.
Cipriani etal. Sleep disturbances and dementia. Psychogeriatrics 2015 Mar;15(1):65-74
Shenker JI, and Singh G Sleep and Dementia Mo Med Jul-Aug 2017;114(4):311-315
Semeredi S, Stajer V, Ostojic J, Vranes M, Ostojic SM. Guanidinoacetic acid with creatine compared with creatine alone for tissue creatine content, hyperhomocysteinemia, and exercise performance: A randomized, double-blind superiority trial. Nutrition. 2019 Jan;57:162-166. doi: 10.1016/j.nut.2018.04.009. Epub 2018 May 17. PMID: 30170305.
Ostojic SM, Ostojic J, Drid P, Vranes M, Jovanov P. Dietary guanidinoacetic acid increases brain creatine levels in healthy men. Nutrition. 2017 Jan;33:149-156. doi: 10.1016/j.nut.2016.06.001. Epub 2016 Jun 8. PMID: 27497517.
Ostojic SM, Ostojic J. Dietary guanidinoacetic acid does not accumulate in the brain of healthy men. Eur J Nutr. 2018 Dec;57(8):3003-3005. doi: 10.1007/s00394-017-1600-2. Epub 2017 Dec 19. PMID: 29255931.
Ferri CP, Prince M, Brayne C, et al; Alzheimer’s Disease International. Global prevalence of dementia: A Delphi consensus study.
M., Kancheva, D., Jordanova, A., & Ivanova, M. (2016). Creatine Deficiency Syndrome could be Missed Easily: A Case Report of Guanidinoacetate Methyltransferase Deficiency Presented with Neurodevelopmental Delay, Seizures, and Behavioral Changes, but Normal Structural MRI. Annals of clinical and laboratory science, 46(5), 557–561.
Stöckler S, Holzbach U, Hanefeld F, Marquardt I, Helms G, Requart M, et al. Creatine deficiency in the brain: a new, treatable inborn error of metabolism. Pediatr Res. 1994;36(3):409-13.
Mercimek-Mahmutoglu S, Stoeckler-Ipsiroglu S, Adami A, Appleton R, Araujo HC, Duran M, et al. GAMT deficiency: features, treatment, and outcome in an inborn error of creatine synthesis. Neurology. 2006;67:480–4.
Stockler-Ipsiroglu S, vanKarnebeek CD. Cerebral creatine deficiencies: a group of treatable intellectual developmental disorders. Semin Neurol. 2014;34(3):350-6.
Mercimek-Mahmutoglu S, Ndika J, Kanhai W, deVillemeur TB, Cheillan D, Christensen E, et al. Thirteen new patients with guanidinoacetatemethyltransferase deficiency and functional characterization of nineteen novel missense variants in the GAMT gene. HumMutat. 2014;35(4):462-9.
Ostojic SM, Ostojic J, Drid P, Vranes M. Guanidinoacetic acid versus creatine for improved brain and muscle creatine levels: a superiority pilot trial in healthy men. Appl Physiol Nutr Metab. 2016 Sep;41(9):1005-7. doi: 10.1139/apnm-2016-0178. Epub 2016 Jun 13. PMID: 27560540.
Zhang etal, 2016 Decreased levels of vitamin B12 in aging, autism and schizophrenia. PMID: 26799654
O'Rourke DJ, Ryan S, Salomons G, Jakobs C, Monavari A, King MD. Guanidinoacetatemethyltransferase (GAMT) defi[ciency: late onset of movement disorder and preserved expressive language. DevMedChildNeurol. 2009;51:404–7.
Caldeira Araújo H, Smit W, Verhoeven NM, Salomons GS, Silva S, Vasconcelos R, Tomás H, TavaresdeAlmeida I, Jakobs C, Duran M. Guanidinoacetate methyltransferase deficiency identified in adults and a child with mental retardation. Am J MedGenet A. 2005;133A:122–
Lion-François L, Cheillan D, Pitelet G, Acquaviva-Bourdain C, Bussy G, Cotton F, et al. High frequency of creatine deficiency syndromes in patients with unexplained mental retardation. Neurology. 2006;67:1713–4..
Mercimek-Mahmutoglu S, Salomons GS. Creatine Deficiency Syndromes. 2009 Jan 15 [Updated 2015 Dec 10]. In: Pagon RA, Adam MP, Ardinger HH,et al.,editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2016. Available from: http://www.ncbi.nlm.nih.gov/books/NBK3794/
Leuzzi V, Mastrangelo M, Battini R, Cioni G.Inborn errors of creatine metabolism and epilepsy. Epilepsia. 2013;54(2):217-27.
Schulze A, Hoffmann GF, Bachert P, Kirsch S, Salomons GS, Verhoeven NM, et al. Presymptomatic treatment of neonatal guanidinoacetate methyltransferase deficiency. Neurology. 2006;67:719–21.
Verbruggen KT, Sijens PE, Schulze A, Lunsing RJ, Jakobs C, Salomons GS, et al. Successful treatment of a guanidinoacetate methyltransferase deficient patient: findings with relevance to treatment strategy and pathophysiology. MolGenetMetab. 2007b;91:294–6.
Morris AA, Appleton RE, Power B, Isherwood DM, Abernethy LJ, Taylor RW, et al. Guanidinoacetate methyltransferase deficiency masquerading as a mitochondrial encephalopathy. J Inherit Metab Dis. 2007; 30(1):100.
Item CB, Mercimek-Mahmutoglu S, Battini R, Edlinger-Horvat C, Stromberger C, Bodamer O, et al. Characterization of seven novel mutations in seven patients with GAMT deficiency. Hum Mutat. 2004;23:524.
Vance, D. E., Walkey, C. J., & Cui, Z. (1997). Phosphatidylethanolamine N-methyltransferase from liver. Biochimica et biophysica acta, 1348(1-2), 142–150. https://doi.org/10.1016/s0005-2760(97)00108-2<
Stanciu, G. D., Luca, A., Rusu, R. N., Bild, V., Beschea Chiriac, S. I., Solcan, C., Bild, W., & Ababei, D. C. (2019). Alzheimer's Disease Pharmacotherapy in Relation to Cholinergic System Involvement. Biomolecules, 10(1), 40. https://doi.org/10.3390/biom10010040
Grossberg S. (2017). Acetylcholine Neuromodulation in Normal and Abnormal Learning and Memory: Vigilance Control in Waking, Sleep, Autism, Amnesia and Alzheimer's Disease. Frontiers in neural circuits, 11, 82. https://doi.org/10.3389/fncir.2017.00082
Perry E. (1988). Acetylcholine and Alzheimer's disease. The British journal of psychiatry : the journal of mental science, 152, 737–740. https://doi.org/10.1192/bjp.152.6.737
Dumas, J. A., & Newhouse, P. A. (2011). The cholinergic hypothesis of cognitive aging revisited again: cholinergic functional compensation. Pharmacology, biochemistry, and behavior, 99(2), 254–261. https://doi.org/10.1016/j.pbb.2011.02.022
Bartus, R. T., Dean, R. L., 3rd, Beer, B., & Lippa, A. S. (1982). The cholinergic hypothesis of geriatric memory dysfunction. Science (New York, N.Y.), 217(4558), 408–414. https://doi.org/10.1126/science.7046051
Carpentieri, A., Marchionatti, A., Areco, V., Perez, A., Centeno, V., & Tolosa de Talamoni, N. (2014). Antioxidant and antiapoptotic properties of melatonin restore intestinal calcium absorption altered by menadione. Molecular and cellular biochemistry, 387(1-2), 197–205. https://doi.org/10.1007/s11010-013-1885-2
Tasdemir, S., Parlakpinar, H., Vardi, N., Kaya, E., & Acet, A. (2013). Effect of endogen-exogenous melatonin and erythropoietin on dinitrobenzene sulfonic acid-induced colitis. Fundamental & clinical pharmacology, 27(3), 299–307. https://doi.org/10.1111/j.1472-8206.2011.01016.x
Parisotto, E. B., Vidal, V., García-Cerro, S., Lantigua, S., Wilhelm Filho, D., Sanchez-Barceló, E. J., Martínez-Cué, C., & Rueda, N. (2016). Chronic Melatonin Administration Reduced Oxidative Damage and Cellular Senescence in the Hippocampus of a Mouse Model of Down Syndrome. Neurochemical research, 41(11), 2904–2913. https://doi.org/10.1007/s11064-016-2008-8
Necefli, A., Tulumoğlu, B., Giriş, M., Barbaros, U., Gündüz, M., Olgaç, V., Güloğlu, R., & Toker, G. (2006). The effect of melatonin on TNBS-induced colitis. Digestive diseases and sciences, 51(9), 1538–1545. https://doi.org/10.1007/s10620-005-9047-3
Trotta, R. J., Lemley, C. O., Vonnahme, K. A., & Swanson, K. C. (2021). Effects of nutrient restriction and melatonin supplementation from mid-to-late gestation on maternal and fetal small intestinal carbohydrase activities in sheep. Domestic animal endocrinology, 74, 106555. https://doi.org/10.1016/j.domaniend.2020.106555
Li, J., Li, R. X., Liu, G., Lv, C. F., Mi, Y. L., & Zhang, C. Q. (2017). Effect of melatonin on renewal of chicken small intestinal mucosa. Poultry science, 96(8), 2942–2949. https://doi.org/10.3382/ps/pex085
Desbonnet etal, 2012 Physiological and behavioural responsivity to stress and anxiogenic stimuli in COMT-deficient mice - PubMed (nih.gov)
Pertegal et al, 2016 Fetal Val108/158Met catechol-O-methyltransferase (COMT) polymorphism and placental COMT activity are associated with the development of preeclampsia - PubMed (nih.gov)
Matos etal. 2016 Epistatic Interaction of CYP1A1 and COMT Polymorphisms in Cervical Cancer - PubMed (nih.gov)
Richdale etal, 2023 Pathways to anxiety and depression in autistic adolescents and adults. Depression and Anxiety Volume 2023 | Article ID 5575932 |
Methylating Enzymes
From Wikipeida N-methyltransferase - Wikipedia
N-methyltransferase may refer to:
-
(RS)-1-benzyl-1,2,3,4-tetrahydroisoquinoline N-methyltransferase
-
3-hydroxy-16-methoxy-2,3-dihydrotabersonine N-methyltransferase
-
(ribulose-bisphosphate carboxylase)-lysine N-methyltransferase
Copyright © 2018 B12 Oils. All Rights Reserved.
Reproduction in whole or in part in any form or medium without express written
permission is prohibited