
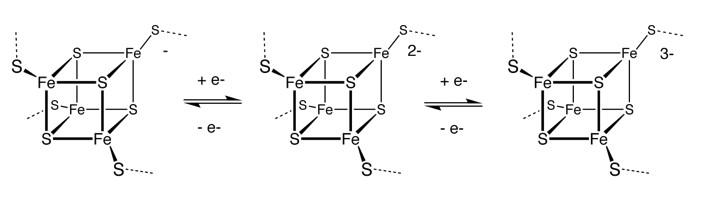
Methylcobalamin and formation of Iron-sulphur proteins
Iron-sulphur
proteins are a group of non-heme, iron containing proteins, in which elemental
iron is complexed with sulphur.
They are characterized by the
presence of iron-sulfur clusters containing sulfide-linked di-, tri-, and
tetra-iron centers in variable oxidation states. They are some of the most
primitive structures in the body and evolved when the earth was devoid of oxygen
and energy transfer was mainly through iron and sulphur complexes. Notable
examples include the Krebs cycle proteins, aconitase and succinate
dehydrogenase, plus ferrodoxin (adrenodoxin - involved in activation of vitamin
D) and complex I, II and III of the electron transport chain. In addition
iron-sulphur complexes are present in enzymes responsible for structure,
including lysyl-hydroxylase
Iron-sulphur
complexes
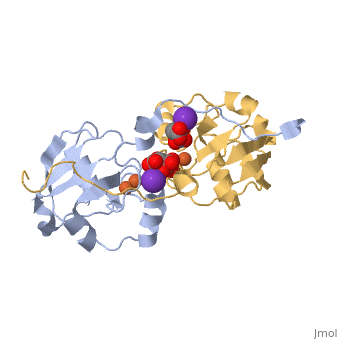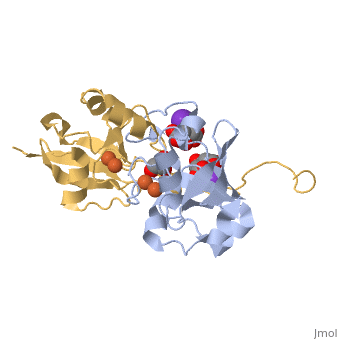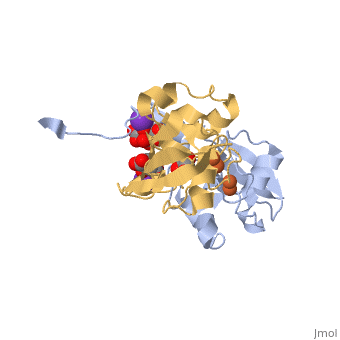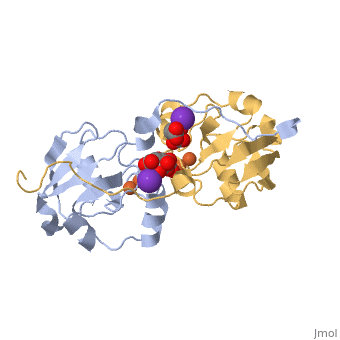
Iron-sulphur
protein, Adrenodoxin. Note the iron-sulphur
complexes
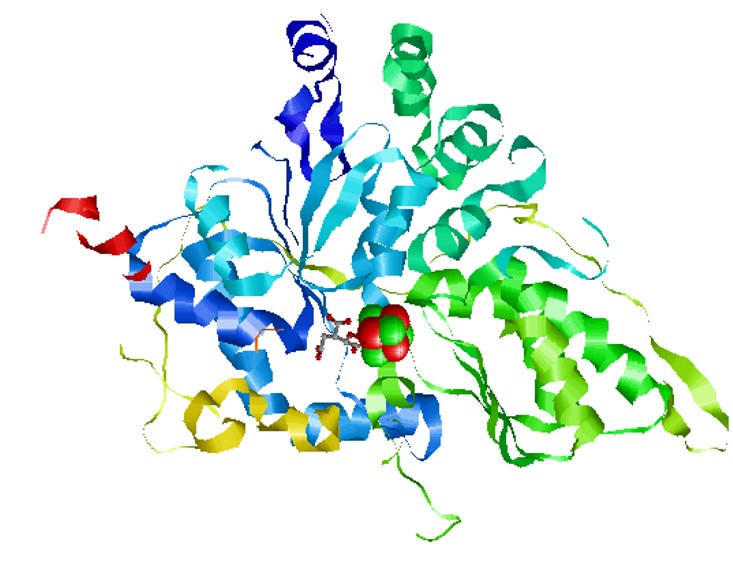
Iron-sulphur
protein, aconitase. Note the iron-sulphur
complexes
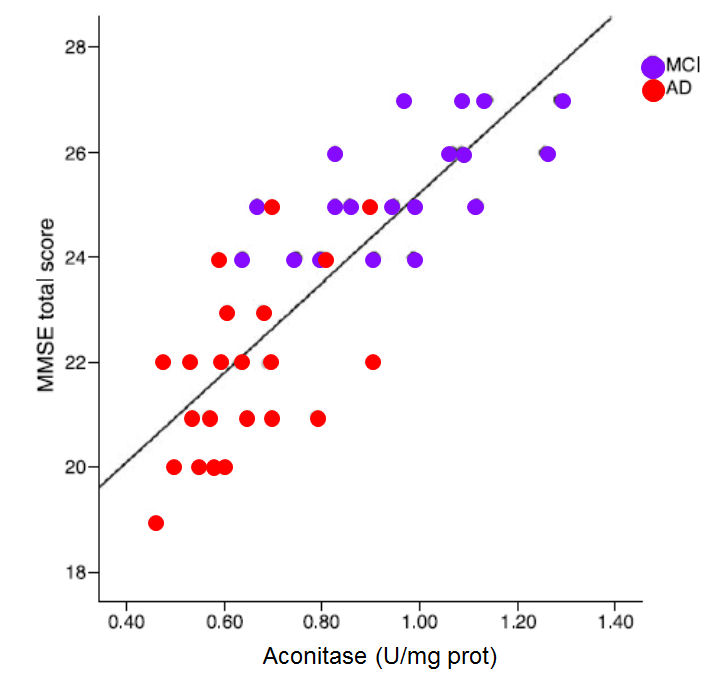
Reduced iron deficiency, particularly when coupled with vitamin B12 deficiency
is associated with lower activity of the Krebs cycle enzyme, Aconitase, which in
turn results in lower energy entering Krebs cycle, higher urinary citrate, and
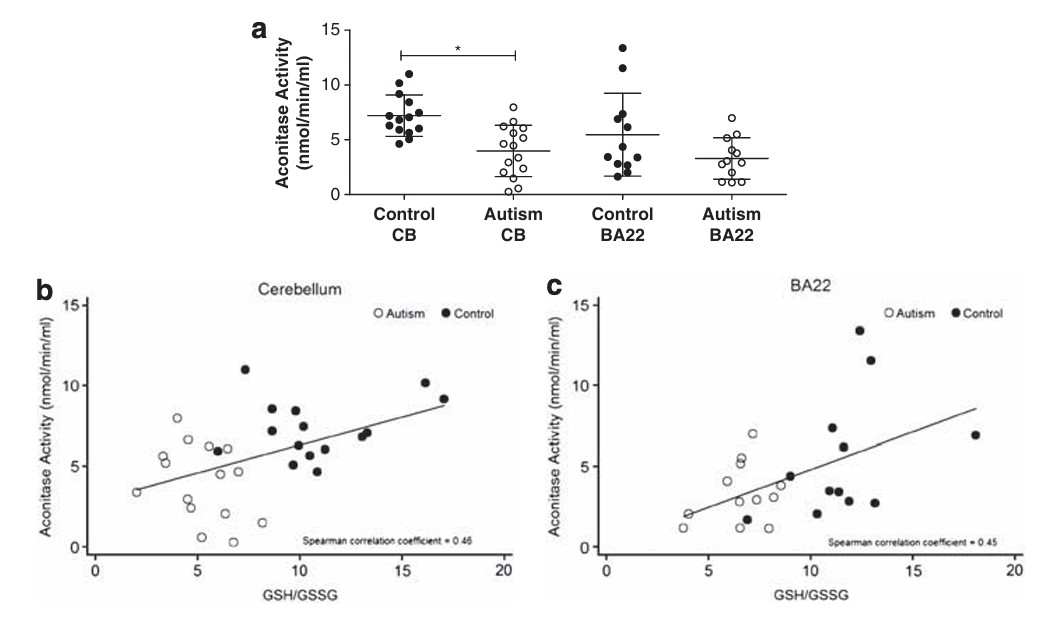
lower Mini Mental score. Lower aconitase activity has been found in the
cerebellum and Brodmann areas of the brains in those with autism.
MMSE score against
the activity of the enzyme aconitase (Figure. Data from Mangialasche etal,2015)
Reduced Aconitase activity in the cerebellum and Brodman area of the brain in
control and those with autism (Fig from Rose etal, 2012). Note the
reduced ratio of GSH:GSSG between those with autism and the NT controls Iron has a major
role in the conversion of energy generated from the
metabolism of fats, sugar and protein, and as iron levels drop (as measured by
serum ferritin), the energy conversion via the iron-sulpur enzyme aconitase
drops rapidly and there is an increased excretion of citrate in urine -
representing metabolic energy that cannot be processed. Iron deficiency in the
diet is an environmental disaster, as it represents a highly inefficient use of
consumed calories, with some children "wasting" upwards of 80% of consumed
calories. The corollary to this is that the highly energy dependent neurons in
the brain are effectively starved of energy as iron levels drop. This then
results in impaired mitochondrial respiration, lower intracellular ATP
production, and leads to chronic neuronal energy insufficiency (Raichle
and Gusnard, 2002), with a subsequent reduction in mitochondrial speed with
reduced hippocampal neuronal development (Bastian etal, 2019). Decreasing levels
of ferritin in serum have been correlated with higher levels of "wasted" citrate
in urine - Personal obvervations)
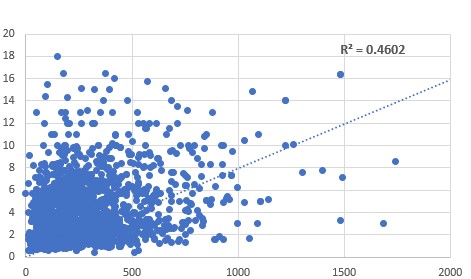
Relationship between serum ferritin (vertical axis) and urinary citrate
(horizontal axis). One of the standard
markers of Methyl B12 deficiency is homocysteine. Urinary Organic Acids testing
(OAT) has shown a correlation between functional vitamin B12 deficiency and
elevations in the neurotransmitter metabolite, HVA. Because OAT does not measure
homocysteine, HVA has been used as a surrogate marker for HVA (see
https://b12oils.com/b12.htm)
Formation of CoQ10 requires 3 methylation reactions. In functional methyl B12
deficiency, levels of the CoQ10 precursor HMG are elevated, and energy transfer
along the electron transport chain is reduced. As can be seen from the
scattergram, the levels of the B12 deficiency marker, HVA correlated with those
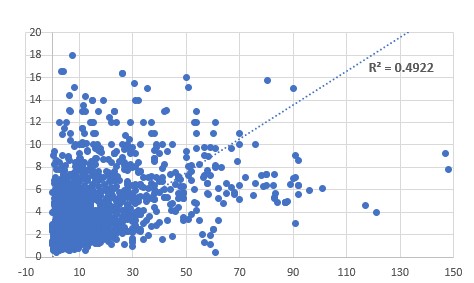
of the CoQ10 deficiency marker, HMG . Relationship between
HVA (vertical axis) and HMG (horizontal axis)
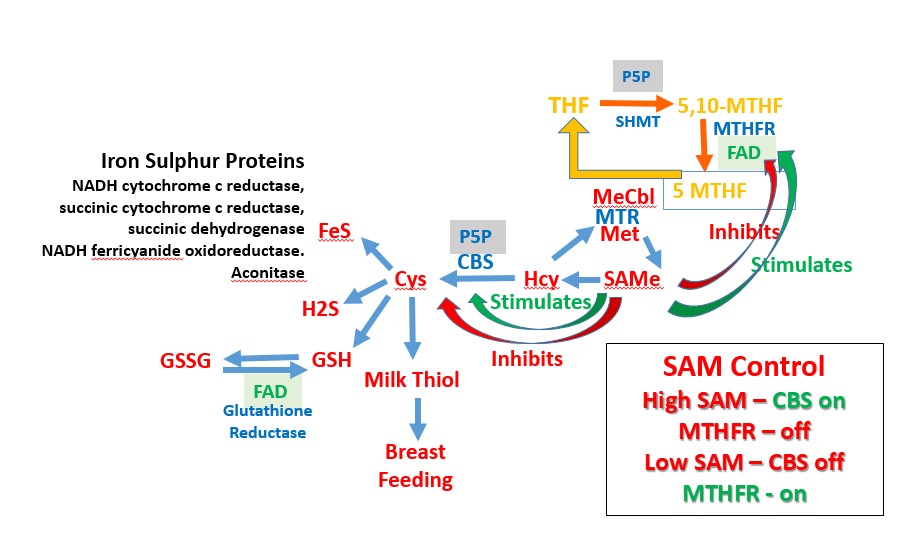
Methyl B12 also has an indirect association with the formation of iron-sulphur
proteins. Hence the sulphur from methionine is the dietary source of the sulphur
in cysteine, glutathione, and in iron-sulphur proteins, such as aconitase and
succinate dehydrogenase. Movement of the sulphur in methionine into the
sulphation pathway requires stimulation of the enzyme, cystathionine beta
synthase (CBS), by S-Adenosylmethionine. In conditions of low methylation due
to Methyl B12 deficiency, the activity of CBS is reduced, and so too is the
movement of sulphur into the sulphation pathway, and there is a reduction in the
capacity to make iron-sulphur proteins, due to a sulphur deficiency.
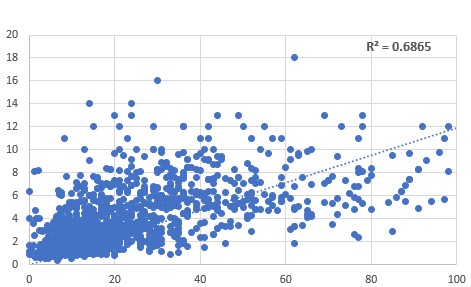
Comparison
between HVA (vertical axis) and citrate (horizontal axis) showed a correlation
between increasing HVA and increasing citrate. Suggesting that methyl B12
deficiency results in lower production of, or lower activity of aconitase. It
should be noted, however, that there will be a co-dependency upon levels of iron
and sulphur from methionine intake, thus reducing the R2 value.
Relationship between
HVA (vertical axis) and citrate (horizontal axis) Similarly, methyl B12 deficiency
also resulted in lower activity of the iron-sulphur protein succinate
dehydrogenase. Increased Methyl B12 deficiency, as represented by increasing HVA
(verical axis), resulted in increased succinic acid (horizontal axis).
Relationship between
HVA (vertical axis) and succinate (horizontal axis) Conversion of 25-OH
vitamin D, to the active form, 1,25-diOHvitamin D, requires a complex
interaction between the enzymes alpha-hydroxylase, and the iron sulphur protein,
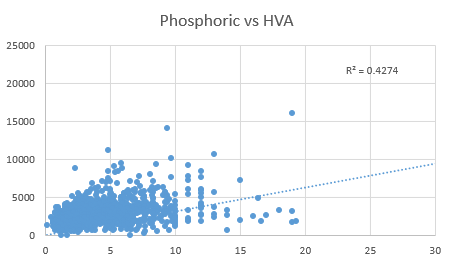
adrenodoxin, and the FAD-dependent enzyme adrenodoxin reductase. Deficiency in active vitamin D can be
estimated by an increase in phosphoric acid. Decreased methyl B12, as estimated
by HVA levels (horizontal axis), resulted in reduced activation of 25-OH-D to
1,25 diOH D, with reduced production of calcium-phosphate resulting in increased
levels of phosphoric acid in urine (vertical axis).
Reduced activation
of vitamin D as determined by an increase in urinary phosphoric acid (vertical
axis) when compared with HVA (Horizontal axis) Production of
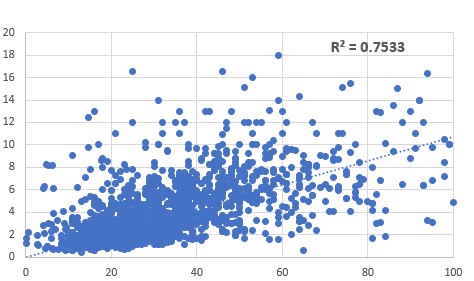
glutathione requires the conjugation of glutamate, cysteine and glycine. In
methyl B12 deficiency, the lower activity of the enzyme cystathionine beta
synthase (CBS) results in reduced
transfer of the sulphur in homocysteine into the sulphation cycle, with a
resultant reduction in the levels of cysteine. In the absence of cysteine, the
molecule, glutathioine (GluCysGly) cannot form and pyroglutamate is formed.
Methyl B12 deficiency as determined by increased HVA (vertical axis) was
correlated with a marked n increase in levels of urinary pyroglutamate
(horizontal axis).
Reduced production
of GSH in methyl B12 deficiency. Methyl B12 deficiency, as determined from
increased urinary HVA (vertical axis) corrleated with an increase in
pyroglutamic acid (horizontal axis)
The iron-sulphur protein, lysine hydroxylase,
functions to modify lysyl residues in proteins such as collagen, allowing proper
hydrogen-bonding of neighbouring strands of collagen, thereby strengthening the
structure. Reduced lysyl-hydroxylase activity is associated with laxity of
joints such as occurs in Ehlers Danlos syndrome, and in many children with
autism. Summary Reduced levels of methyl B12 have been associated
with lower energy transfer in the electron transport chain, due to lower
production of the electron shuttle vector, CoQ10. Reduced levels of methyl B12 have been associated a
reduced production of iron-sulphur proteins, resulting in lower activity of the
Krebs cycle enzymes aconitase and succinate dehydrogenase. Reduced levels of methyl B12 have been associated
with a lower production of the intracellular anti-oxidant and disulphide shuttle
vector, glutathione, and an increased production of pyroglutamic acid. Reduced levels of methyl B12 have been associated
with lower activity of lysine hydroxylate and an increased laxity of joints.
Ferriera etal. Multilevel impacts of iron in the brain" The cross talk between
neurophysiological mechanisms, cognition, and social behaviour. Pharmaceuticals
2018 12
https://www.ncbi.hlm.nih.gov/pmc/articles/PMC6789770/
Yusrawati etal Differences in brain-derived neurotrophic factor between neonates
born to mothers with normal and low ferritin. Asia Pac J Clin Nutr 2018 27:
389-392 Schmidt et al
Maternal intake of supplemental iron and risk of autism spectrum disorder 2014
PMID 25249546 Radlowski and
Johnson Perinatal iron deficiency and neurocognitive development Front Hum
Neurosci 2013 7, 585
Rao and Georgieff Perinatal aspects of iron
metabolism
https://www.ncbi.nim.nih.gov/pubmed/12477276 Amin etal In utero
iron status and auditory neural maturation in premature infants as evaluated by
auditory brainstem response. PMID 19939407
Domellöf M. (2013). Iron and other micronutrient deficiencies in low-birthweight
infants. Nestle
Nutrition Institute workshop series, 74,
197–206.
https://doi.org/10.1159/000348772
Wiegersma, A. M., Dalman, C., Lee, B. K., Karlsson, H., & Gardner, R. M. (2019).
Association of Prenatal Maternal Anemia With Neurodevelopmental Disorders. JAMA
psychiatry, 76(12),
1294–1304.
https://doi.org/10.1001/jamapsychiatry.2019.2309
Berglund, S., & Domellöf, M. (2014). Meeting iron needs for infants and
children. Current
opinion in clinical nutrition and metabolic care, 17(3),
267–272.
https://doi.org/10.1097/MCO.0000000000000043
Hare
etal, 2013 A delicate balance: Iron metabolism and diseases of the brain...
PMIC 3715022
Kwik-Uribe etal,
2000 Chronic marginal iron intakes during early development in mice result in
persistent changes in dopamine metabolism and myelin composition.PMID
11053527
Choudhury etal 2015 Latent iron deficiency at birth influences auditory neural
maturation in late preterm and term infants
PMID 26310540
Armony-Sivan etal 2004 Iron status and neurobehavioral development of premature
infants
PMID 15318248
Mercer JS, Erickson-Owens DA, Deoni SCL, Dean DC 3rd, Collins J, Parker AB, Wang
M, Joelson S, Mercer EN, Padbury JF. Effects of Delayed Cord Clamping on 4-Month
Ferritin Levels, Brain Myelin Content, and Neurodevelopment: A Randomized
Controlled Trial. J Pediatr. 2018 Dec;203:266-272.e2. doi:
10.1016/j.jpeds.2018.06.006. Epub 2018 Jul 6. PMID: 30473033; PMCID: PMC6259583.
Wenger etal, 2017 Effect of iron deficiency on simultaneous measures of
behavior, brain activity and energy..
PMID 28784049
Zumbrennen-Bullough etal 2014 Abnormal brain iron metabolism in Irp2 deficient
mice is associated with mild neurological and behavioral impairments
PMID 24896637
Georgieff 2008
The role of iron in neurodevelopment: fetal iron deficiency and the developing
hippocampus
PMID PMC2711433
Georgieff MK,
Krebs NF, Cusick SE. The Benefits and Risks of Iron Supplementation in Pregnancy
and Childhood. Annu Rev Nutr. 2019 Aug 21;39:121-146. doi:
10.1146/annurev-nutr-082018-124213. Epub 2019 May 15. PMID: 31091416; PMCID:
PMC7173188.
Wang M. Iron Deficiency and Other Types of Anemia in Infants and Children. Am
Fam Physician. 2016 Feb 15;93(4):270-8. PMID: 26926814.
Sherjil etal 2010
Iron deficiency anaemia - a risk factor for febrile seizures in children
PMID 22338422
Fallah etal 2014
Iron deficiency and iron deficiency anemia in children with first attack of
seizure and on healthy control group....
PMC PMC4135276
Gillberg etal
2017 Febrile seizures and epilepsy: Association with autism and other
neurodevelopmental disorders in the child.......
PMID 28754226
McCue etal 2016
Prevalence of non-febrile seizures in children with idiopathic autism spectrum
disorder and their unaffected siblings.......
PMID 27894273
Kabakus etal 2002 Reversal of iron deficiency anemia-induced
peripheral neuropathy by iron treatment of children with iron deficiency anemia. PMID:
12200980
Siddappa etal 2007 The assessment of newborn iron stores
atbirth: a review of teh literature and standards for ferritin concentrations
Mangialasche etal, 2015 Lymphocytic mitochondrial aconitase
activity is reduced in Alzheimer's disease and mild cognitive impairment. PMID:
25322927
Pawlak etal, 2016. Iron status of
Vegetarian Adults: A literature review.
Iron-sulphur proteins
Energetics
of Iron Deficiency
Energetics
of Methyl B12 Deficiency
 .
.
Reduced
activation of vitamin D in Methyl B12 Deficiency
Reduced
production of Glutathione in Methyl B12 Deficiency
Methyl B12
and Lysyl-hydroxylase
References
Hara 2007 Autism and epilepsy: a retrospective follow-up study PMID 17321709
Fallah etal 2016 Evaluation efficacy of ferrous sulfate therapy on headaches of 5-15 years old iron deficient children with migraine PMC
Giulivi et al Mitochondrial dysfunction in autism. J. Am. Med Assoc 2010 304: 2387-2396
Chauhan etal Brain region-specific deficit in mitochondrial electron transport chain complexes in children with autism. J. Neurochem. 2011 117: 209-22
Tang et al Mitochondrial abnormalities in temporal lobe of autistic brain. Neurobiol Dis 2013 54: 349-361
Goh et al Mitochondrial dysfunction as a neurobiological subtype of Autism Spectrum Disorder.. JAMA psychiatry 2014 71: 665-671
Napoli et al Deficits in bioenergetics and impaired immune response in granulocytes from children with autism. Pediatrics 2014 133:e1405-10
Zumbrennen-Bullough; et al. Abnormal brain iron metabolism in Irp2 deficient mice is associated with mild neurological and behavioural impairment. PloS One 2014 e98072 PMC 4045679
Sherjil et al Iron deficiency anaemia - a risk factor for febrile seizures in children 2010 PMID 22338422
Westmark Soy infant formula and seizures in children with autism: a retrospective study. 2007 PMID 24622158
de Sa et al. Anemia in pregnancy: impact of weight and in the development of anemia in the newborn. Nutr Hosp 2015 32:2071-2079
Gaspar et al. Relationships between iron status in pregnant women and their newborn babies.. Acta Obstet Gynecol. Scand. 1993 72:5234-7
Jaime-Perez et al. Sub-optimal fetal iron acquisition under a maternal environment. Arch Med Res. 2005 36: 598-602
Shao et al. Maternal serum ferritin concentraion is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. J. Nutri. 2012 142:2004-9
Lee et al. Prevalence of anaemia and associations between neonatal iron status, hepcidin and maternal iron status among neonates born to pregnant adolescents. Ped Res. 2016 79:42-8
Kabakus et al, Reversal of iron deficiency anemia-induced peripheral neuropathy by iron treatment of children with iron deficiency anemia. J Trop Ped 2002 48: 204-9
https://nutritionovereasy.com/2015/05/cast-iron-pans-can-increase-your-iron-intake/
Brittin HC, Nossaman CE. Iron content of food cooked in iron utensils. J Am Diet Assoc. 1986;86:897–901
Junqueira-Franco etal, Iron absorption from beans with different contents of iron, evaluated by stable isotopes.
Eftekhari etal The relationship between iron status and thyroid hormone concentration in iron-deficient adolescent Iranian girls. https://www.ncbi.nlm.nih.gov/pubmed/16500878
Lopez, R., Micoulaud Franchi, J. A., Chenini, S., Gachet, M., Jaussent, I., & Dauvilliers, Y. (2019). Restless legs syndrome and iron deficiency in adults with attention-deficit/hyperactivity disorder. Sleep, 42(5), zsz027. https://doi.org/10.1093/sleep/zsz027
Veltri etal, 2016 Prevalence of thyroid autoimmunity and dysfunction in women with iron-deficiency in early pregnancy... https://www.ncbi.nlm. nih.gov/pubmed/27450694
Li etal, 2016 The relationship between iron deficiency and thyroid function in Chinese women during early pregnancy https://www.jstage.jst.go.jp/article/jnsv/62/6/62_392/_article
Tenq etal, 2018 Iron deficiency may predict greater risk for hypthyroxinemia: A retropective cohort study of pregnant women in China https://www.ncbi.nlm.nih.gov/pubmed/29968513
Maldonado-Araque etal, 2018 Iron deficiency is associated with hypothyroxinemia ... https://www.ncbi.nlm.nih.gov/pubmed/29700318
Yu et al Iron deficiency, an independent risk factor for isolated hypothyroxinemia in pregnant ...... https://www.ncbi.nlm.nih.gov/pubmed/25599388
Hu etal Perinatal iron deficiency-induced hypothyroxinemia impairs early brain development https://www.ncbi.nlm.nih.gov/pubmed/27231981
Name etal Iron Bisglycinate Chelate and Polymaltose Iron for the Treatment of Iron Deficiency Anemia: A Pilot Randomized Trial PMID 6416187
Milman et al. Body iron and individual iron prophylaxis in pregnancy - should the iron dose be adjusted according to serum ferritin. Ann Hematol. 2006 85: 567-73.
Gabis LV, Shaham M, Leon Attia O, Shefer S, Rosenan R, Gabis T, Daloya M. The Weak Link: Hypotonia in Infancy and Autism Early Identification. Front Neurol. 2021 Feb 4;12:612674. doi: 10.3389/fneur.2021.612674. PMID: 33613430; PMCID: PMC7891038
Lopez-Espejo MA, Nuńez AC, Moscoso OC, Escobar RG. Clinical characteristics of children affected by autism spectrum disorder with and without generalized hypotonia. Eur J Pediatr. 2021 Oct;180(10):3243-3246. doi: 10.1007/s00431-021-04038-7. Epub 2021 Apr 14. PMID: 33855616.
Oslejskova H, Dusek L, Makovska Z, Rektor I. Epilepsia, epileptiform abnormalities, non-right-handedness, hypotonia and severe decreased IQ are associated with language impairment in autism. Epileptic Disord. 2007 Dec;9 Suppl 1:S9-18. doi: 10.1684/epd.2007.0154. PMID: 18319196
Fillano JJ, Goldenthal MJ, Rhodes CH, Marín-García J. Mitochondrial dysfunction in patients with hypotonia, epilepsy, autism, and developmental delay: HEADD syndrome. J Child Neurol. 2002 Jun;17(6):435-9. doi: 10.1177/088307380201700607. PMID: 12174964.
Casanova EL, Baeza-Velasco C, Buchanan CB, Casanova MF. The Relationship between Autism and Ehlers-Danlos Syndromes/Hypermobility Spectrum Disorders. J Pers Med. 2020 Dec 1;10(4):260. doi: 10.3390/jpm10040260. PMID: 33271870; PMCID: PMC7711487.
Baeza-Velasco C, Cohen D, Hamonet C, Vlamynck E, Diaz L, Cravero C, Cappe E, Guinchat V. Autism, Joint Hypermobility-Related Disorders and Pain. Front Psychiatry. 2018 Dec 7;9:656. doi: 10.3389/fpsyt.2018.00656. PMID: 30581396; PMCID: PMC6292952.
Tedla JS, Asiri F, Alshahrani MS, Gular K. Hypermobility among children with autism spectrum disorders and its correlation with anthropometric characteristics. J Pak Med Assoc. 2021 Apr;71(4):1076-1080. doi: 10.47391/JPMA.436. PMID: 34125746.
Kindgren E, Quińones Perez A, Knez R. Prevalence of ADHD and Autism Spectrum Disorder in Children with Hypermobility Spectrum Disorders or Hypermobile Ehlers-Danlos Syndrome: A Retrospective Study. Neuropsychiatr Dis Treat. 2021 Feb 10;17:379-388. doi: 10.2147/NDT.S290494. PMID: 33603376; PMCID: PMC7882457.
Casanova EL, Sharp JL, Edelson SM, Kelly DP, Casanova MF. A Cohort Study Comparing Women with Autism Spectrum Disorder with and without Generalized Joint Hypermobility. Behav Sci (Basel). 2018 Mar 17;8(3):35. doi: 10.3390/bs8030035. PMID: 29562607; PMCID: PMC5867488.
Buterbaugh A, Mroczkowski HJ, Shankar SP, Visootsak J. Contribution of Family History in Co-occurring Down Syndrome and Ehlers-Danlos Syndrome. Ann Paediatr Rheumatol. 2013 Apr 1;2(4):165-167. doi: 10.5455/apr.081820131456. PMID: 24839582; PMCID: PMC4020180.
Aljaadi etal. Suboptimal biochemical riboflavin status is associated with lower hemoglobin and higher rates of anemia in a sample of Canadian and Malaysian women of reproductive age.J Nutrit, 2019
Ars etal 2019 Prenatal folate, homocysteine and vitamin B12 levels and child brain volumes. Br J. Nutr. 122: S1-S9.
Rose S, Melnyk S, Pavliv O, Bai S, Nick TG, Frye RE, James SJ. Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl Psychiatry. 2012 Jul 10;2(7):e134. doi: 10.1038/tp.2012.61. PMID: 22781167; PMCID: PMC3410618
Peyrin-Biroulet L, Williet N, Cacoub P. Guidelines on the diagnosis and treatment of iron deficiency across indications: a systematic review. Am J Clin Nutr. 2015 Dec;102(6):1585-94. doi: 10.3945/ajcn.114.103366. Epub 2015 Nov 11. PMID: 26561626.
Fava et al. Heart failure and iron deficiency 2019 https://www.ncbi.nlm.nih.gov/pubmed/30821294
Jankowska et al Iron deficiency predicts impaired exercise capacity in patients with cystolic chronic heart failure https://www.ncbi.nom.nih.gov/pubmed/22041326
https://www.thewomens.org.au/images/uploads/fact-sheets/Iron-Infusion-0718.pdf
Ramirez, J. O., Cabrera, S. A., Hidalgo, H., Cabrera, S. G., Linnebank, M., Bassetti, C. L., & Kallweit, U. (2013). Is preeclampsia associated with restless legs syndrome?. Sleep medicine, 14(9), 894–896. https://doi.org/10.1016/j.sleep.2013.03.013
Minár, M., Košutzká, Z., Habánová, H., Rusňák, I., Planck, K., & Valkovič, P. (2015). Restless legs syndrome in pregnancy is connected with iron deficiency. Sleep medicine, 16(5), 589–592. https://doi.org/10.1016/j.sleep.2014.11.023
H
Sim, J.J.; Lac, P.T.; Liu, I.L.A.; Meguerditchian, S.O.; Kumar, V.A.; Kujubu, D.A.; Rasgon, S.A. Vitamin D deficiency and anemia: A cross-sectional study. Ann. Hematol. 2010, 89, 447–452.
Blanco-Rojo, R.; Pérez-Granados, A.M.; Toxqui, L.; Zazo, P.; de la Piedra, C.;
Vaquero, M.P. Relationship between vitamin D deficiency, bone remodelling and
iron status in iron-deficient young women consuming
an iron-fortified food. Eur. J. Nutr. 2013, 52, 695–703.
Lee, J.A.; Hwang, J.S.; Hwang, I.T.; Kim, D.H.; Seo, J.-H.; Lim, J.S. Low vitamin D levels are associated with both iron deficiency and anemia in children and adolescents. Pediatr. Hematol. Oncol. 2015, 32, 99–108.
Liu, T.; Zhong, S.; Liu, L.; Liu, S.; Li, X.; Zhou, T.; Zhang, J. Vitamin D deficiency and the risk of anemia: A meta-analysis of observational studies. Ren. Fail. 2015, 37, 929–934.
Constantini, N.W.; Arieli, R.; Chodick, G.; Dubnov-Raz, G. High prevalence of vitamin D insufficiency in athletes and dancers. Clin. J. Sport Med. 2010, 20, 368–371.
Sharma, S.; Jain, R.; Dabla, P.K. The role of 25-hydroxy vitamin D deficiency in iron deficient children of North India. Indian J. Clin. Biochem. 2015, 30, 313–317.
Bener A, Khattab AO, Bhugra D, Hoffmann GF. Iron and vitamin D levels among autism spectrum disorders children.Ann Afr Med. 2017 Oct-Dec;16(4):186-191. doi: 10.4103/aam.aam_17_17.
Kaymak Cihan M, Ünver Korğalı E. Is there an association between vitamin D level
and iron deficiency in children?Arch Argent Pediatr. 2018 Dec
1;116(6):e736-e743. doi: 10.5546/aap.2018.eng.e736.
PMID: 30457722
Malczewska-Lenczowska J, Sitkowski D, Surała O, Orysiak J, Szczepańska B, Witek
K.The Association between Iron and Vitamin D Status in Female Elite
Athletes.Nutrients. 2018 Jan 31;10(2):167. doi: 10.3390/nu10020167.
PMID: 29385099
Nur-Eke R, Özen M.The Relationship between Vitamin D Levels and Iron Deficiency
and Anemia in Adults Applied for Periodic Medical Examination.
Clin Lab. 2020 Jun 1;66(6). doi: 10.7754/Clin.Lab.2019.190918.
Uwaezuoke SN. Vitamin D deficiency and anemia risk in children: a review of emerging evidence.Pediatric Health Med Ther. 2017 May 10;8:47-55. doi: 10.2147/PHMT.S129362. eCollection 2017.
Raichle, M. E., & Gusnard, D. A. (2002). Appraising the brain's energy budget. Proceedings of the National Academy of Sciences of the United States of America, 99(16), 10237–10239. https://doi.org/10.1073/pnas.172399499
Bastian, T. W., von Hohenberg, W. C., Georgieff, M. K., & Lanier, L. M. (2019). Chronic Energy Depletion due to Iron Deficiency Impairs Dendritic Mitochondrial Motility during Hippocampal Neuron Development. The Journal of neuroscience : the official journal of the Society for Neuroscience, 39(5), 802–813. https://doi.org/10.1523/JNEUROSCI.1504-18.2018
Bastian, T. W., Rao, R., Tran, P. V., & Georgieff, M. K. (2020). The Effects of Early-Life Iron Deficiency on Brain Energy Metabolism. Neuroscience insights, 15, 2633105520935104. https://doi.org/10.1177/2633105520935104
Benson, C. S., Shah, A., Frise, M. C., & Frise, C. J. (2021). Iron deficiency anaemia in pregnancy: A contemporary review. Obstetric medicine, 14(2), 67–76. https://doi.org/10.1177/1753495X20932426
Frayne J, Pinchon D. Anaemia in pregnancy. Aust J Gen Pract. 2019 Mar;48(3):125-129. doi: 10.31128/AJGP-08-18-4664. PMID: 31256475.
http://www.ncbi.nlm.nih.gov/pubmed/25599388
http://www.ncbi.nlm.nih.gov/pubmed/25150119
http://www.ncbi.nlm.nih.gov/pubmed/12487769
http://www.ncbi.nlm.nih.gov/pubmed/12097675
Copyright © 2018 B12 Oils. All Rights Reserved.
Reproduction in whole or in part in any form or medium without express written
permission is prohibited