
Ehlers-Danlos Syndrome and Vitamin B12
-
Ehlers-Danlos Syndromes are a group of connective tissue disorders.
-
Generally EDS involves joint hypermobility, skin hyperextensibility, and tissue fragility
-
The frequency of EDS is quoted as 1 in 2,500 to 1 in 5,000
-
EDS, or EDS-like conditions appear to be much more frequent in people with vitamin B12 deficiency
-
Collagen cross-linking requires hydroxylation of collagen by Prolyl-hydroxylase and Lysyl-hydroxylase
-
Both hydroxylases require singlet iron atoms in their active site.
-
Generation of singlet iron is dependent upon functional vitamin B2 and vitamin B12
-
80% of people with EDS have POTS
-
Vitamin B12 deficiency has been linked to POTS
EDS and HSD
EDS (Ehler-Danlos Syndrome) is one of a number of the Hypermobility disorders, which includes the Hypermobility Spectrum Disorders (HSD), hypermobility EDS (EDS) and some rarer EDS (GEDS) disorders (Arthrochalasia, Kyphoscoliotic EDS, and Dermatospraxis EDS), the later of which are primarily genetic in origin. The majority of Spectrum of HDS and EDS, however, have so far not been found to have an identified genetic defect, even though many cases are more common within families.
Role of Collagen in EDS
The hypermobility and hyperextensibility and tissue fragility typical in EDS appears to relate to structural defects in the collagen that comprises the skin and the tendons of the joints. Such defects lead to the production of weaker and less rigid collagen. These defects can also affect the digestive system, which contains a high proportion of connective tissue.
Structure of collagen
Collagen fibres are arranged in an alpha helical fashion similar to the double helix of DNA, except collagen has 3 strands wound around each other in similar fashion to the strands in a piece of rope. In order for the protein to form the helix two helix forming amino acids, proline and glycine predominate in the sequence. A special form of proline, hydroxyproline constitutes around 14% of collagen. The 3 strands of the collagen fibre are then linked by hydrogen bonds formed between hydroxyproline residues on adjacent strands of collagen. In addition some forms of collagen also have an hydroxylated form of lysine as part of their structure.
Production of hydroxyproline requires a specific iron-dependent enzyme, prolyl-hydroxylase, and production of hydroxy-lysine requires lysyl-hydroxylase.As can be seen from the cartoon, collagen is extensively modified with hydroxyproline (Blue - Glycine; White - Proline; Red - hydroxyproline)
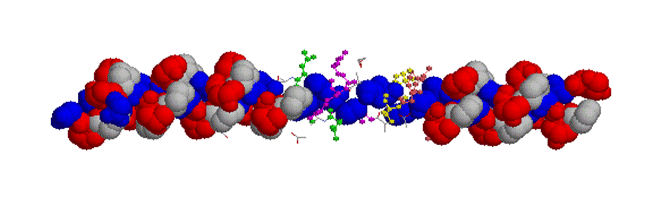
Compare this to collagen that is not modified with hydroxyproline (Blue - Glycine; White - Proline; Red - hydroxyproline)
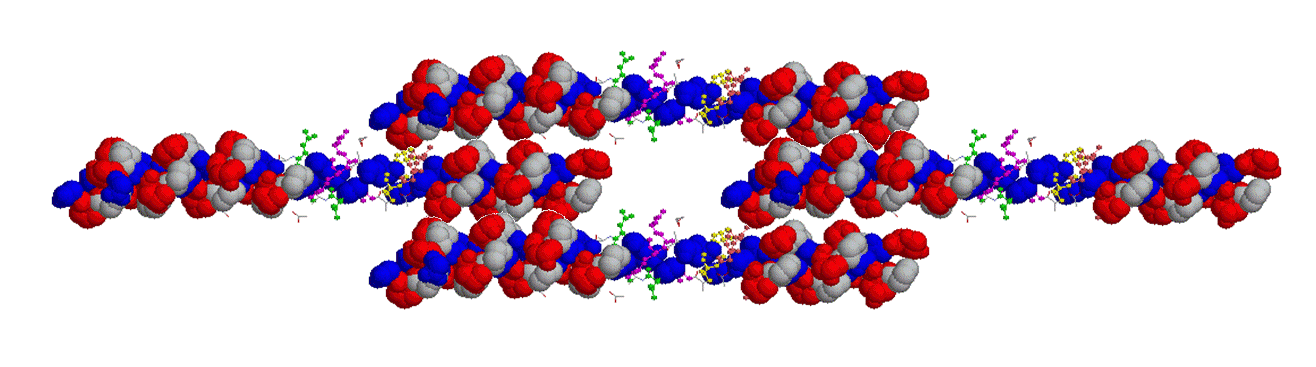
Hydrogen bonding of adjacent collagen chains via hydroxyproline (Blue - Glycine; White - Proline; Red - hydroxyproline)

Alternative Staggered Hydrogen bonding of adjacent collagen chains via hydroxyproline (Blue - Glycine; White - Proline; Red - hydroxyproline). Note how neighbouring hydroxyproline residues can align and so form H-bonds, and retain a rigid yet flexible structure.
In some but not all collagen types hydroxylysine is also present (Blue - Glycine; White - Proline; Red - hydroxyproline; Pink - hydroxylysine)
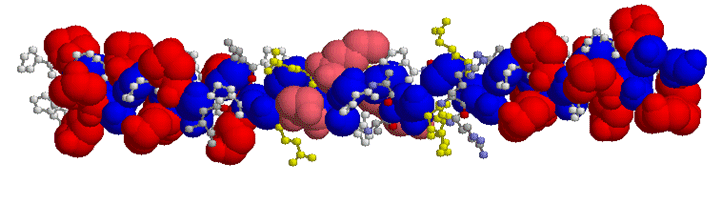
Hydrogen-bonds between molecules of collagen help to both align the collagen molecules but also to strengthen the structure and act a little like compounds such as "Velcro". Additional "strength" of collagen is obtained by hydroxylation of lysine and subsequent cross-linking of adjacent hydroxylysine- residues
Lack of hyrogen-bonds creates a much less rigid and floppy structure that does not associate strongly between neighbouring collagen molecules. This would be analagous to when the hooks where off a "Velcro" zip. This then potentially can reduce the strength of ligaments dramatically. Experimentally this can be observed by a lower "melting point" temperature of non-hydroxylated collagen, in comparison to normal collagen. Properly formed collagen, with its full complement of hydroxy-proline and hydroxylysine cross-links has a tensile strength, which is greater than steel of a similar diameter.
(see https://www.ncbi.nlm.nih.gov/boos/NBK21582/
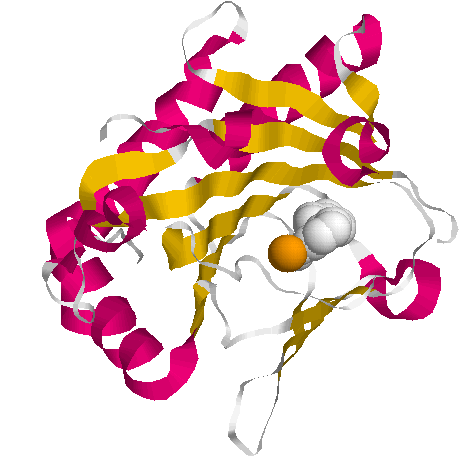
Activity of Prolyl Hydroxylase
The function of collagen prolyl hydroxylase is dependent upon the two co-factors, vitamin C and iron. Lack of vitamin C is associated with the development of scurvy due to lack of activity of this enzyme. It is also associated with poor wound healing, easy bruising, gingivitis, nosebleeds, myalgia and arthralgia. Low vitamin C is also associated with the production of weaker collagen (DePhillipo etal, 2018; Boverra etal, 1998)

Activity of Lysyl Hydroxylase
The function of collagen Lysyl hydroxylase is also dependent upon iron. Mutations in the gene for lysyl-hydroxylase has been found in some individuals with genetically transmitted EDS.
Functional Iron Deficiency due to vitamin B2 deficiency
Functional vitamin B2 is critical for iron processing in the body, starting with reduction of Fe+++ to Fe++ in the intestine, and to later the release of transferrin-bound Fe+++ within cells. Vitamin B2, as FAD, also is essential for maintaining reduced glutathione with in cells to further add in the solubilization of Fe+++ and conversion to Fe++. Deficiency of reduced glutathione can therefore lead to precipitation of insoluble Fe+++ within cells and a reduced ability to make enzymes containing iron in their active centers, such as prolyl hydroxylase and lysyl hydroxylase. Hence individuals with non-functional B2 are more likely to produce non-hydroxylated collagen, with the result that the rigidity of the normally H-bonded collagen will be reduced thereby increasing the likelihood of hEDS.
Functional Iron Deficiency due to vitamin B12 deficiency
Functional vitamin B2 is critical for maintaining adequate levels of methyl Co(III)B12 within the methylation cycle and ultimately for the production of S-Adenosylmethionine. This in turn is essential for the ultimate conversion of methionine to homocysteine, and the subsequent formation of cysteine, and for the production of glutathione, which is essential for intracellular processing of iron.. Functional vitamin B12 deficiency, therefore can in itself lead to lower levels of intracellular glutathione and hence contribute to the lack of activity of lysyl and prolyl hydroxylases, thereby contributing to the development of "lax collagen" typically described in hEDS.
EDS and POTS
The association of vitamin B12 deficiency and EDS may explain the observation that 80% of people with EDS have POTS. A recent study in children found that vitamin B12 levels were significantly lower in children with POTS (47.2%) when compared to those without (18%) (Oner etal, 2014).
Gastrointestinal issues with EDS
Various intestinal symptoms have been associated with EDS/HDS, which vary from person to person, and include acid reflux, constipation, abdominal bloating, abdominal discomfort. It is, however, hard to determine if these symptoms are a feature of EDS/HDS alone, or if they are features of the potential deficiencies that cause EDS/HDS
Vascular EDS
Disorders of collagen formation can also affect the vasculature, and cause vascular EDS. This is associated with fragile blood vessels, collapsed lung, and heart valve problems.
Current Concepts on EDS
Data suggests that there are two main types of EDS
Type 1 (Classic) EDS. Genetically determined EDS. In this form of EDS there is a heritable defect in either the structure or sequence of one form of collagen, or there is defect in the activity of prolyl-hydroxylase or lysyl-hydroxylase. Persons having this form of EDS will have had it from birth. The incidence of type I EDS has changed little in recent times. The condition will be basically irreversible. These would be the rarer and more severe forms of EDS. The condition is associated nclude hypotonia with delayed motor development, fatigue and muscle cramps, and easy bruising (Malfait et al, 2007; Levy, 2004).
Type 2 EDS. Environmentally determined EDS. In this from of EDS some environmental or developmental factor changes the availability of iron, functional vitamin B2, or functional vitamin B12. Persons having this form of EDS seem to develop it either in their teenage years, or somewhat later in their 30s or 40s. The resultant deficiency or availability of iron affects the function of prolylhydroxylase and/or lysyl-hydroxylase, leading to a reduced level of hydroxylation of proline and/or lysine, and thereby leading to much weaker strength of collagen, which ultimately causes more frequent dislocations of joints or a higher frequency of ligament damage. The condition potentially is more frequent in women, and the frequency is increasing with the decrease in iron in the community and the increased rate of functional B2 and functional B12 deficiency. The condition is potentially reversible given the proper nutrients.
Type 3 EDS. Ehlers-Danlos syndrome type arthrochalasia (aEDS) is a rare genetic disease characterized by severe generalized joint hypermobility, bilateral congenital hip dislocation, skin hyperextensibility, muscle hypotonia, and mild dysmorphic features (Martin-Martin et al, 2022).
Type VI EDS. Also classified as the kyphoscoliotic type, are clinically characterized by neonatal kyphoscoliosis, generalized joint laxity, skin fragility, and severe muscle hypotonia at birth (Yeowell and Walker, 2000).The condition is characterized by a deficiency in Lysyl Hydroxylase enzyme, normally hydroxylates specific lysine residues in the collagen molecule to form hydroxylysines which have two important functions. The residues serve as attachment sites for galactose and glucosylgalactose and they also act as precursors of the hydrogen-bonding crosslinking process that gives collagen its tensile strength (Arun et al, 2006; Rohrbach et al, 2011).
Generally the Hypermobility is associated with muscle hypotonia (Mintz-Itkin etal, 2009; Yis et al, 2007).
Extrapolation from Concepts on EDS
The observation that the iron dependent enzymes Prolyl hydroxylase and Lysyl hydroxylase are essential for building strength into collagen fibres and hence into structural compenents such as ligaments, potentially explains the greater propensity for persons with lower iron intake, such as many young girls, to have greater sports associated injuries than their male counterparts, purely due to their lower iron intake. Of particular note is the 4-8 fold higher ACL injury rate in females playing soccer, as opposed to males (Kirkendall etal, 2002).
References
Dr Alan J. Akim Utube - https://www.youtube.com/watch?v=S3pvhudR7cE&list=PLJ8LTDV_7QkvNCk1sYXY8IqzajPbtpPYr
DePhillipo etal, 2018 Efficacy of Vitamin C Supplementation on Collagen Synthesis and Oxidative Stress After Musculoskeletal Injuries: A Systematic Review PMC 6204628
Bovera etal 1998 Effect of vitamin C and its derivatives on collagen synthesis and cross-linking by normal human fibroblasts PMID 18505499.
Oner etal Postural orthostatic tachycardia syndrome (POTS) and vitamin B12 deficiency in adolescents.
PMID 24366986Malfait F, Wenstrup R, De Paepe A. Classic Ehlers-Danlos Syndrome. 2007 May 29 [updated 2018 Jul 26]. In: Adam MP, Everman DB, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2022. PMID: 20301422.
Levy HP. Hypermobile Ehlers-Danlos Syndrome. 2004 Oct 22 [updated 2018 Jun 21]. In: Adam MP, Everman DB, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2022. PMID: 20301456.
Martín-Martín M, Cortés-Martín J, Tovar-Gálvez MI, Sánchez-García JC, Díaz-Rodríguez L, Rodríguez-Blanque R. Ehlers-Danlos Syndrome Type Arthrochalasia: A Systematic Review. Int J Environ Res Public Health. 2022 Feb 7;19(3):1870. doi: 10.3390/ijerph19031870. PMID: 35162892; PMCID: PMC8835098.
Arun T, Nalbantgil D, Sayinsu K. Orthodontic treatment protocol of Ehlers-Danlos syndrome type VI. Angle Orthod. 2006 Jan;76(1):177-83. doi: 10.1043/0003-3219(2006)076[0177:OTPOES]2.0.CO;2. PMID: 16448289.
Mintz-Itkin R, Lerman-Sagie T, Zuk L, Itkin-Webman T, Davidovitch M. Does physical therapy improve outcome in infants with joint hypermobility and benign hypotonia? J Child Neurol. 2009 Jun;24(6):714-9. doi: 10.1177/0883073808329526. Epub 2009 Apr 7. PMID: 19351812.
Yeowell HN, Walker LC. Mutations in the lysyl hydroxylase 1 gene that result in enzyme deficiency and the clinical phenotype of Ehlers-Danlos syndrome type VI. Mol Genet Metab. 2000 Sep-Oct;71(1-2):212-24. doi: 10.1006/mgme.2000.3076. PMID: 11001813.
Rohrbach M, Vandersteen A, Yiş U, Serdaroglu G, Ataman E, Chopra M, Garcia S, Jones K, Kariminejad A, Kraenzlin M, Marcelis C, Baumgartner M, Giunta C. Phenotypic variability of the kyphoscoliotic type of Ehlers-Danlos syndrome (EDS VIA): clinical, molecular and biochemical delineation. Orphanet J Rare Dis. 2011 Jun 23;6:46. doi: 10.1186/1750-1172-6-46. PMID: 21699693; PMCID: PMC3135503.
Yiş U, Dirik E, Chambaz C, Steinmann B, Giunta C. Differential diagnosis of muscular hypotonia in infants: the kyphoscoliotic type of Ehlers-Danlos syndrome (EDS VI). Neuromuscul Disord. 2008 Mar;18(3):210-4. doi: 10.1016/j.nmd.2007.11.006. Epub 2007 Dec 26. PMID: 18155911.
Kirkendal etal 2002 A prospective 3 year injury incidence in your soccer. Med Sci Sports Ex.34:S101
Thanks
Special thanks to Dr Robin Link for the EDS Channel films at https://www.youtube.com/channel/UC652wu-mvi2ghwQN-is7LIQ
Copyright © 2018 B12 Oils. All Rights Reserved.
Reproduction in whole or in part in any form or medium without express written
permission is prohibited