
Dopamine Paradox The close liaison between vitamin B2 and vitamin B12
Functional vitamin B12 deficiency, can be assessed by a number of metabolic markers that increase in deficiency. The commonly used markers are homocysteine and Methyl Malonic Acid (MMA). However, there are several OAT markers that are greatly increased in vitamin B12 deficiency, including HVA, VMA, QA, KA, Pyroglutamic acid, and hydroxymethylglutamic acid. (See B12). Of these, one particular marker is of considerable interest, HVA. HVA is the break-down product of dopamine. and elevated levels of dopamine occur when there is insufficient Methyl B12 to supply the methyl group for the universal methyl donor, SAM. As the degree of vitamin B12 deficiency increases there is a build up of dopamine, which can be measured in OAT by the dopamine degradation product HVA.

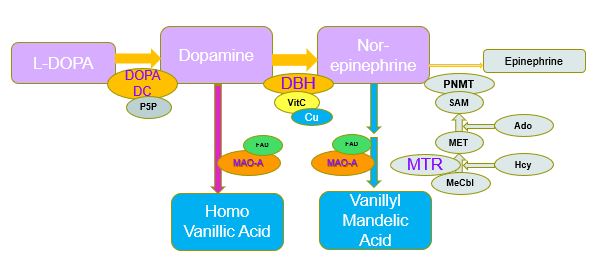
Normal production of Epinephrine (Adrenalin)
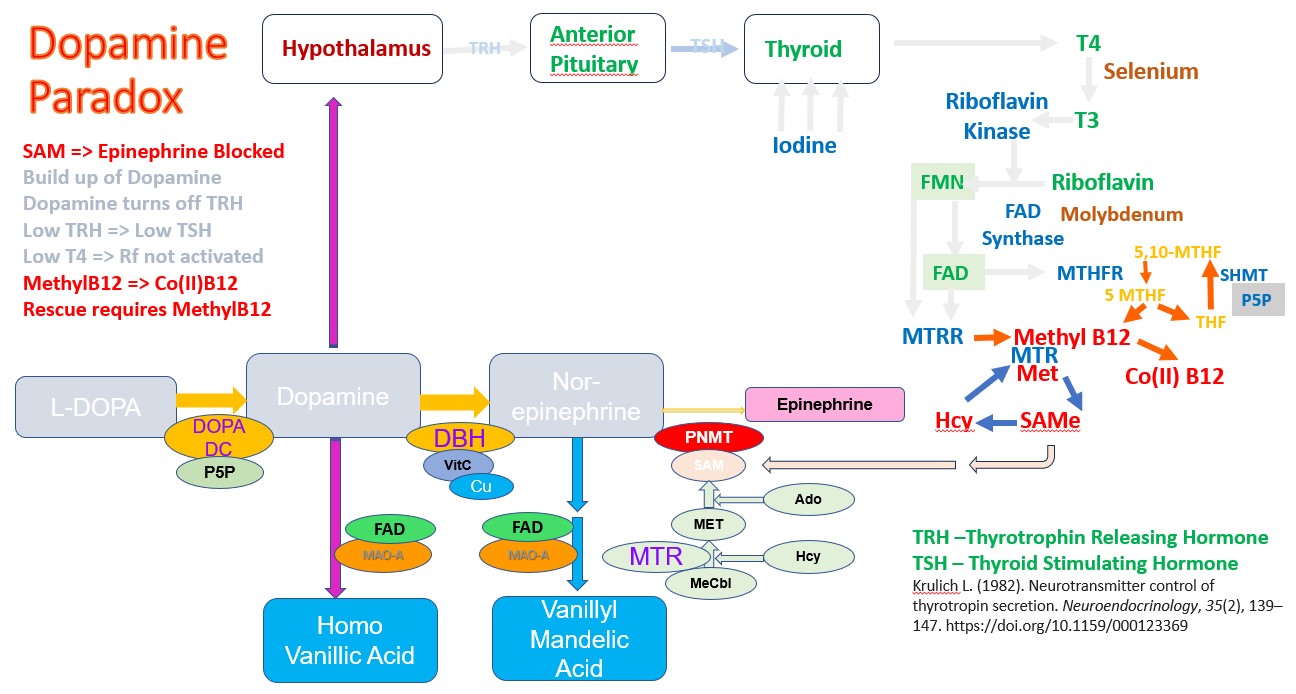
Increased production of Dopamine in Methyl B12 deficiency.
Dopamine Function
Dopamine is a neurotransmitter that is involved in mood, pleasure, and motivation. Dopamine has also been implicated in the rewards associated with learning. Elevations in dopamine are associated with the addictive activity of drugs such as amphetamines, cocaine and opiates. Over-production of dopamine is associated with lack of interest, decreased motivation, poor memory, depression and feelings of hopelessness. Dopamine stimulators such as addictive drugs generally raise dopamine levels three to four times higher than base-line. In Autism, however, dopamine levels, as measured by HVA can commonly be 10 to 20 times and even times times higher than normal.
Dopamine and the Thyroid
It is well known that functional B2 is required for the maintenance of activity the enzymes MTHFR and MTRR, it is perhaps less well known that normal thyroid function is also required for the production of thyroid hormone (T4) and for the product T3. Part of this involves the action of the Hypothalamus, which secretes Thyrotropin releasing hormone (TRH), which then acts upon the Anterior Pituitary to release Thyroid Stimulating Hormone, TSH
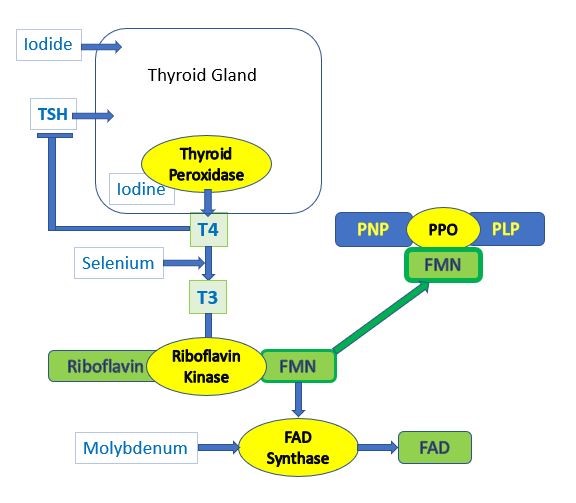
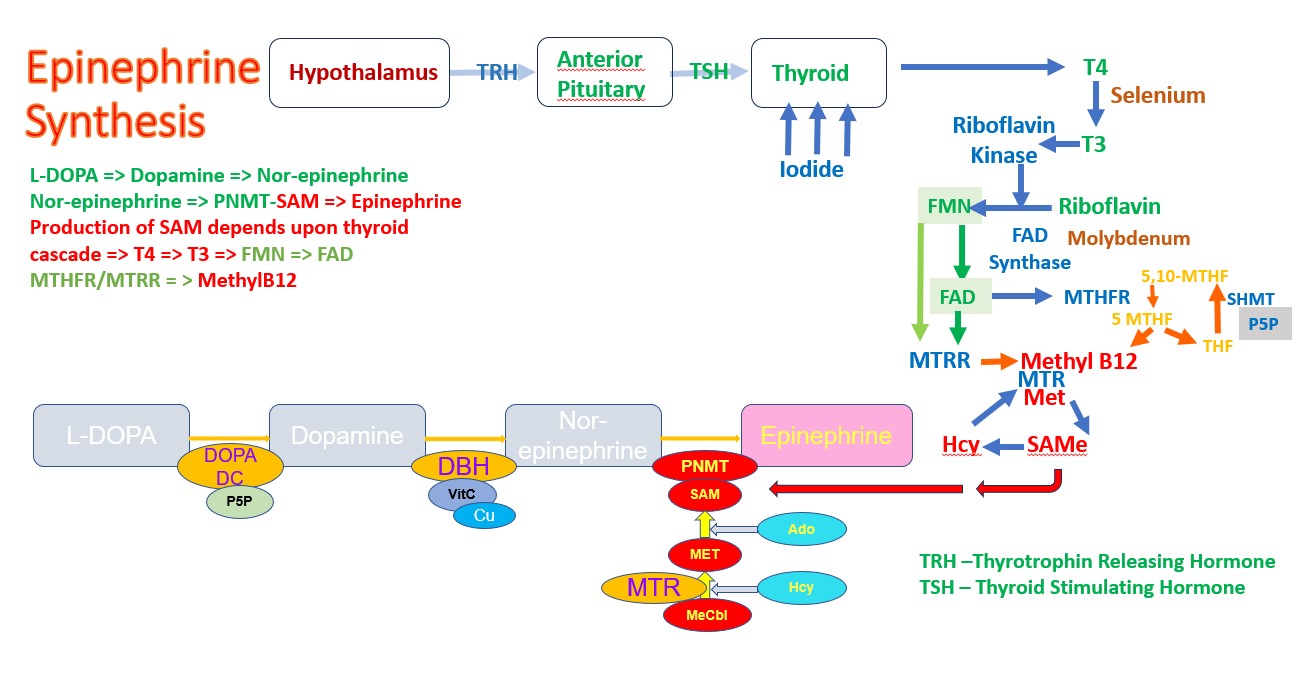
This pathway is regulated in a number of ways, but of note is the down-regulation of the pathway by Dopamine. Hence as dopamine rises the production of TRH is inhibited and in turn so too is TSH. At this stage, it does not matter how much Iodide or Selenite you take, you cannot stimulate higher production of T4, or T3. Effectively when vitamin B12 deficiency is great enough, activation of vitamin B2 is reduced. OAT analysis shows a close correlation between HVA and the CoQ10 deficiency marker HMG, the Glutathione deficiency marker - Pyroglutamic acid, and levels of Phosphoric acid. Elevated phosphoric acid has previously been shown to correlated with Pyroglutamate levels.
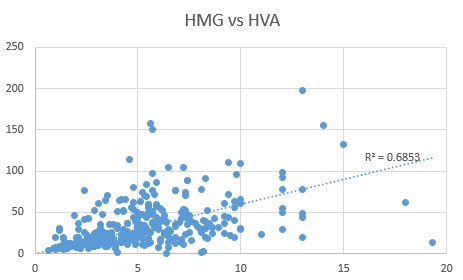
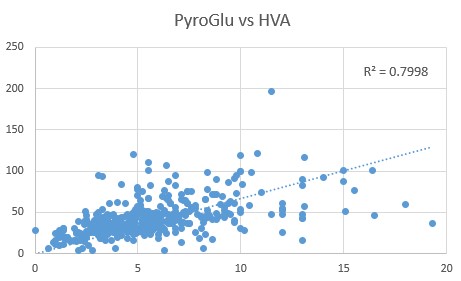
HVA, HMG, PyroGlu and Phosphoric Acid
Comparison of HVA levels to the CoQ10 deficiency marker, HMG, revealed a close correlation (0.6853), as too was the relationship between Pyroglutamic acid and HVA (0.7998)
We have previously shown that there is also a close relationship between levels of Pyroglutamic acid and Phosphoric acid (0.757), and this is further supported by a close relationship between Phosphoric acid and HVA (0.7074).
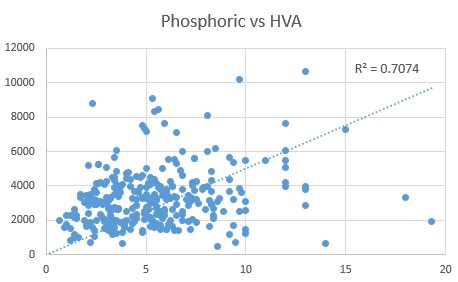
The data obtained from OAT of children with autism, supports the relationship between the elevated HVA, caused by Methyl B12 deficiency and other markers of vitamin B12 deficiency, particularly CoQ10 deficiency (HMG), lack of production of GSH (Pyroglutamate) and observed functional vitamin D deficiency. Given the known Dopamine Paradox, this would strongly support the notion, that in order to fix functional B2 deficiency, as a causative agent for functional B12 deficiency, it would be necessary to supply sufficient Methyl B12, to enable the series of reactions dopamine => Nor-epinephrine => Epinephrine to occur, thereby lowering the levels of Dopamine and thereby "unblocking" the production of TRH, and the subsequent thyroid-mediated cascade of riboflavin activation.
Associated conditions of Methyl B12 Deficiency, Elevated PyroGlu , reduced levels of GSH and reduced production of SeCystNA
An extension to the elevated HVA is the lack of production of GSH - as mentioned above and an alteration in the GSH:GSSG ratio. Reduced methylation causes a reduction in the transfer of the sulphur from homocysteine into the sulphation cycle, leading to lower intracellular cysteine, and reduced production of glutathione. Lack of cysteine then causes an increase in Pyroglutamic acid, one of the surrogate markers for vitamin B12 deficiency. Reduced GSH works in combination with thiosulfate sulphur transferase in the formation of SeCystRNA, and the efficacy of the reaction drops in functional B12 deficiency. In addition levels of toxic intracellular sulphite increase (ASD 107 nmol/ml, NT 2.1 nmol/ml) as well as thiosulfate (ASD 131 nmol/ml, NT 19 nmol/ml) (Kruithof et al, 2020). Cysteine-S-Sulfate becomes elevated as is the cases in AD (Darst etal, 2021).This can then result in a metabolic spiral, as lack of production of SeCystRNA, will reduce the production of Selenoproteins, such as the deiodinases that are responsible for conversion of T4 to T3. This in turn leads to lower production of ribofavin kinase, with a reduced activity of MTHFR and MTRR, which are critical for maintaining the activity of MethylB12. This is yet another reason to treat with increasing doses of Methyl B12.
TSH/T4 feedback in the Dopamine Paradox
Preliminary has shown that in conditions of the Dopamine Paradox, supplementation with higher and higher doses of Iodide - over 3 mg/day, results in little change in T4 levels, however TSH levels slowly increase. In addition, there is no evidence of "fixing" the functional B12 deficiency. Hence, serum creatinine is a break-down product of creatine, and as functional B12 levels decrease, so too does creatinine. It can be seen that despite a 10-fold increase in Iodide concentration, TSH has not dropped, rather it has risen, and there has been no significant change in serum creatinine, which is out of range low. In the graph, Iodide was increased from 600 ug/day to 6 mg/day over a 6 month period.
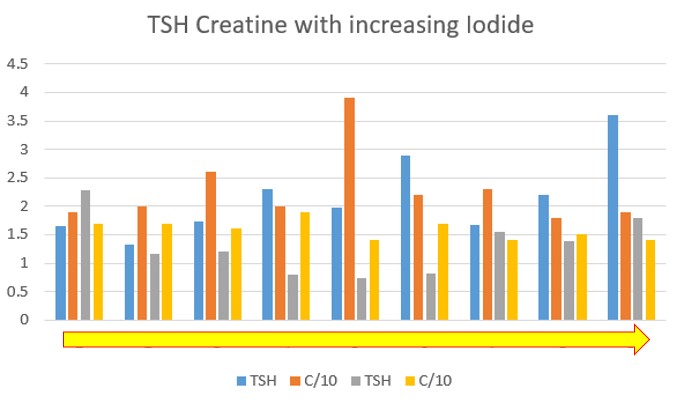
Change in TSH/Creatine with increasing Iodide
Associated genetics of Dopamine Paradox - MTHFR and MTRR and MTR
Preliminary data suggests that those with +/+ mutations in MTHFR, MTRR and MTR are more likely to experience the paradox. Mutations in MTR are known to only respond to increased Methyl B12, as too MTRR, whilst both MTFHR and MTRR require higher levels of FAD for functional activity.
Signs of Dopamine Paradox - creatinine, HVA
Very low serum creatinine is indicative of very poor methylation, whilst HVA that is elevated above 2.5 (5 times normal) is a sign of insufficient Methyl B12. Elevated serum B12, particularly over 1000 pmol/L. Inability to have feedback of elevated Iodide intake and TSH levels. "taking very high amounts of iodide and selenite but numbers keep going the wrong way with very little B12 movement".
The Hypothalamus and the Dopamine Paradox
Elevated dopamine reduces hypothalamus function, which apart from the reduced production of Thyrotropin releasing hormone, also reduces the production of Corticosteroid Releasing Hormone, Gonadotrophin Releasing Hormone, and can affect temperature regulation as well as regulation of thirst and hunger.
Things that are contra in the Dopamine Paradox - vitamin B12 forms
Use of inactive forms of B12, such as hydroxy B12, cyano B12 and also the single use of Adenosyl B12 all will block uptake of methyl B12 and will saturate the B12 binding proteins in serum. High dose oral methyl B12 is inactivated in the stomach and then also competes for B12 binding proteins.
Avoiding the Dopamine Paradox
Given that the driving force behind the Dopamine Paradox is lack of functional Methyl B12, which then drives up the levels of dopamine (and the metabolism product, HVA), it is essential that sufficient "incoming" Methyl B12 is available to keep methylation going.
NB: The liver is the major storage organ for vitamin B12, and so if there is B12, dud or not, it can keep supplying B12 to the body, and hence keep serum B12 levels very high. The body is very good at maintaining levels of vitamin B12, and binds up B12 with two main proteins, one is transcobalamin, which is responsible for uptake of B12 into cells (active or inactive) and the other is haptocorrin, which binds both active and inactive B12. Haptocorrin binds to circulating vitamin B12 and is subsequently taken up by the liver, and also removes circulating vitamin B12, via salivary secretion. Once these two binding proteins are saturated, it is hard to calculate how much B12 is present in the body, as all you have is very elevated serum B12. The haptocorrin-bound material is secreted into the stomach via the Salivary glands, but if it is inactive. The haptocorrin-bound material is then released from Haptocorrin, by the action of bile acids and proteases. The released (inactive B12 -in this case) is bound to intrinsic factor and the IF-B12 is taken up from the gut via the Intrinsic factor receptor, however, the B12 that is taken up is inactive. It then is “passed” to transcobalamin, but as it is inactive it is useless.
In order to circumvent this problem one has to administer large amounts of free active B12 (methyl and adenosyl), either by injection, or by topical administration via TransdermOilTM technology. When serum B12 levels are very high, free vitamin B12 is unlikely to become bound by either HC, or TC in serum because both are saturated. The free vitamin B12 is either excreted into urine or is secreted into bile where it competes with B12 that is in food, or B12 which was bound to HC, and released in the stomach, and so competes with inactive B12. This is part of the Entero-hepatic circulation of vitamin B12 (Guéant et al, 2022: 1984: Willigan etal, 1958; Cooksley and Tavill, 1975; Adams, 1963; Green etal, 1981; 1982; el Kholty et al, 1991). Hence the higher the inactive B12 was in the past, the longer one would have to compete out the inactive. It also means that there has to be a continuous stream of active B12 administered. Preliminary data suggests that at this stage, methyl B12 would be preferable.
Dopamine Paradox in Parkinson's Disease
There is evidence of inhibition of , or alterations in, Hyopthalamic function in Parkinsonian patients resulting in abnormalities of growth hormone release
(Randyk etal, 1987), and autonomic dysfunction..High dose Iodide and the Wolff-Chaikoff Effect
In addition to the effect on the thyroid seen with elevated dopamine, administration of high doses of Iodide can have a similar effect, the Wolff-Chaikoff effect, whereby the thyroid becomes non-responsive to the elevated levels of Iodide. The non-responsiveness of the thyroid to such stimulation is thought to be protective for the thyroid, and to stop damage to the thyroid by the high doses of Iodide.
Copyright.
The descriptions and findings on the Dopamine Paradox, is the property of B12 Oils Pty Ltd. Reproduction in whole or in part constitutes an infringement in the Copyright law. Copyright infringement carries serious penalties.
References
Altered Neurotransmitter Metabolites in vitamin B12 deficiency
Activation of Vitamin D Nexus Theory
Russell-Jones, G. Paradoxical Vitamin B12 Deficiency: Normal to Elevated Serum B12, With Metabolic Vitamin B12 Deficiency. J Biol Today's World, 2022, 11(3), 001-004
Krulich L. (1982) Neurotransmitter control of thyrotropin secretion. Neuroendocrinology, 35, 139-147 .
Duval, F., Mokrani, M. C., Erb, A., Danila, V., Lopera, F. G., Foucher, J. R., & Jeanjean, L. C. (2021). Thyroid axis activity and dopamine function in depression. Psychoneuroendocrinology, 128, 105219. https://doi.org/10.1016/j.psyneuen.2021.105219
Schuiling, G. A., Valkhof, N., Moes, H., & Koiter, T. R. (1993). Dopamine and TRH-induced prolactin secretion in pseudopregnant rats. Life sciences, 53(4), 357–363. https://doi.org/10.1016/0024-3205(93)90754-q
Besses, G. S., Burrow, G. N., Spaulding, S. W., & Donabedian, R. K. (1975). Dopamine infusion acutely inhibits the TSH and prolactin response to TRH. The Journal of clinical endocrinology and metabolism, 41(5), 985–988. https://doi.org/10.1210/jcem-41-5-985
Hanew, K., Utsumi, A., Sugawara, A., Shimizu, Y., & Yoshinaga, K. (1991). Evidence for dopamine-related and TRH-related pituitary TSH and PRL pools in patients with prolactinoma. Acta endocrinologica, 124(5), 545–552. https://doi.org/10.1530/acta.0.1240545
Havranek, T., Bacova, Z., & Bakos, J. (2024). Oxytocin, GABA, and dopamine interplay in autism. Endocrine regulations, 58(1), 105–114. https://doi.org/10.2478/enr-2024-0012
Arias-Carrión, O., & Pŏppel, E. (2007). Dopamine, learning, and reward-seeking behavior. Acta neurobiologiae experimentalis, 67(4), 481–488. https://doi.org/10.55782/ane-2007-1664
Wang, Y., Li, N., Yang, J. J., Zhao, D. M., Chen, B., Zhang, G. Q., Chen, S., Cao, R. F., Yu, H., Zhao, C. Y., Zhao, L., Ge, Y. S., Liu, Y., Zhang, L. H., Hu, W., Zhang, L., & Gai, Z. T. (2020). Probiotics and fructo-oligosaccharide intervention modulate the microbiota-gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder. Pharmacological research, 157, 104784. https://doi.org/10.1016/j.phrs.2020.104784
Mandic-Maravic, V., Grujicic, R., Milutinovic, L., Munjiza-Jovanovic, A., & Pejovic-Milovancevic, M. (2022). Dopamine in Autism Spectrum Disorders-Focus on D2/D3 Partial Agonists and Their Possible Use in Treatment. Frontiers in psychiatry, 12, 787097. https://doi.org/10.3389/fpsyt.2021.787097
Pavăl, D., & Micluția, I. V. (2021). The Dopamine Hypothesis of Autism Spectrum Disorder Revisited: Current Status and Future Prospects. Developmental neuroscience, 43(2), 73–83. https://doi.org/10.1159/000515751
Hellings, J. A., Arnold, L. E., & Han, J. C. (2017). Dopamine antagonists for treatment resistance in autism spectrum disorders: review and focus on BDNF stimulators loxapine and amitriptyline. Expert opinion on pharmacotherapy, 18(6), 581–588. https://doi.org/10.1080/14656566.2017.1308483
Cooper et al., 1983 Dopamine infusion significantly decreased TSH levels in both normal subjects and hypothyroid patients, indicating dopamine's inhibitory effect on TSH secretion. "Dopamine at all doses significantly blunted TRH-stimulated TSH and TSH-beta release, and blunted TRH-mediated alpha release at the two highest dopamine doses." (https://pubmed.ncbi.nlm.nih.gov/6190590/)).
Massara et al., 1978 Dopamine infusion led to significant decreases in plasma TSH and PRL levels across various groups, supporting dopamine's inhibitory role on TSH secretion. (https://pubmed.ncbi.nlm.nih.gov/573767/)
Shupnik MA, Greenspan SL, Ridgway EC. Transcriptional regulation of thyrotropin subunit genes by thyrotropin-releasing hormone and dopamine in pituitary cell culture. J Biol Chem. 1986 Sep 25;261(27):12675-9. PMID: 2427524.
Jin J, Hashizume T. Effects of hypothalamic dopamine on growth hormone-releasing hormone-induced growth hormone secretion and thyrotropin-releasing hormone-induced prolactin secretion in goats. Anim Sci J. 2015 Jun;86(6):634-40. doi: 10.1111/asj.12333. Epub 2014 Nov 30. PMID: 25442325.
Mitsuma T, Hirooka Y, Izeki K, Sin K, Nogimori T. Effects of dopamine on the release of thyrotropin-releasing hormone from the rat retina in vitro. Horm Metab Res. 1992 Jun;24(6):263-5. doi: 10.1055/s-2007-1003309. PMID: 1634191.
Fagin KD, Neill JD. The effect of dopamine on thyrotropin-releasing hormone-induced prolactin secretion in vitro. Endocrinology. 1981 Dec;109(6):1835-40. doi: 10.1210/endo-109-6-1835. PMID: 6796383.
Dieguez C, Foord SM, Peters JR, Hall R, Scanlon MF. Interactions among epinephrine, thyrotropin (TSH)-releasing hormone, dopamine, and somatostatin in the control of TSH secretion in vitro. Endocrinology. 1984 Mar;114(3):957-61. doi: 10.1210/endo-114-3-957. PMID: 6141935.
Enjalbert A, Guillon G, Mouillac B, Audinot V, Rasolonjanahary R, Kordon C, Bockaert J. Dual mechanisms of inhibition by dopamine of basal and thyrotropin-releasing hormone-stimulated inositol phosphate production in anterior pituitary cells. Evidence for an inhibition not mediated by voltage-dependent Ca2+ channels. J Biol Chem. 1990 Nov 5;265(31):18816-22. PMID: 1699937.
Cui ZJ, Gorelick FS, Dannies PS. Calcium/calmodulin-dependent protein kinase-II activation in rat pituitary cells in the presence of thyrotropin-releasing hormone and dopamine. Endocrinology. 1994 May;134(5):2245-50. doi: 10.1210/endo.134.5.8156928. PMID: 8156928.
Mitsuma T, Sun DH, Nogimori T, Chaya M, Ohtake K, Hirooka Y. Dopamine inhibits thyrotropin-releasing hormone release from rat adrenal gland in vitro. Horm Res. 1987;25(4):223-7. doi: 10.1159/000180656. PMID: 3108135.
Darst BF, Huo Z, Jonaitis EM, Koscik RL, Clark LR, Lu Q, Kremen WS, Franz CE, Rana B, Lyons MJ, Hogan KJ, Zhao J, Johnson SC, Engelman CD. Metabolites Associated with Early Cognitive Changes Implicated in Alzheimer's Disease. J Alzheimers Dis. 2021;79(3):1041-1054. doi: 10.3233/JAD-200176. PMID: 33427733; PMCID: PMC8054536.
Sandyk, R., Iacono, R. P., & Bamford, C. R. (1987). The hypothalamus in Parkinson Disease. The Italian Journal of Neurological Sciences, 8(3), 227-234. https://doi.org/10.1007/BF02337479
Copyright © 2024 B12 Oils. All Rights Reserved.
Reproduction in whole or in part in any form or medium without express written
permission is prohibited