
Vitamin B12 Deficiency and Parkinson's Disease
-
Parkinson's disease (PD) is an aging-related movement disorder mainly caused by a deficiency of neurotransmitter dopamine
-
PD is clinically characterized by tremors, rigidity, slowness of movement, and postural imbalance.
-
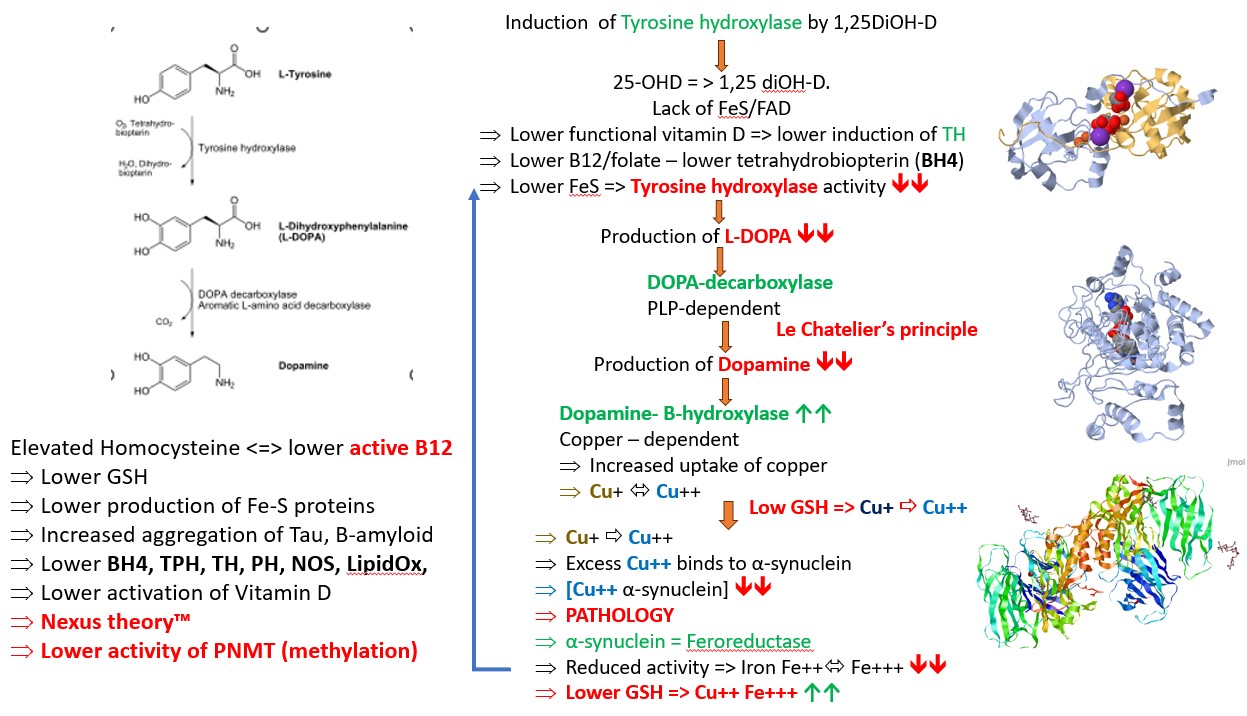
Production of dopamine requires the production of L-DOPA by the iron-dependent enzyme tyrosine hydroxylase
-
Selenium is essential for the activation of vitamin B2
-
Lower Selenium is found in the brains of those with Parkinson's disease
-
Lower functional vitamin B2 leads to lower functional vitamin B12
-
Lower functional vitamin B12 is associated with higher serum homocysteine levels
-
Lower functional vitamin B12 is associated with lower GSH:GSSG ratio, potentially causing Tau and Beta Amyloid pathology
-
Severity of Parkinson's disease correlates with increased homocysteine levels
-
Reduction of iron in the brain requires the action of alpha-synuclein.
-
Altered alpha-synuclein is a feature of Parkinson's disease
-
Iron precipitation is a feature of neurodegnerative diseases such as Parkinson's disease
-
Vitamin D is essential for differentiation of neuronal stem cells
-
Activation of vitamin D in the brain requires a multi-step process with iron, B2 and B12 involved
-
Lower vitamin D is correlated with a higher rate of Parkinson's
-
Low vitamin D is associated with reduced gross motor skills and fine motor skills.
-
Lack of vitamin D has been associated with reduced motivation, emotion, learning ability and memory
-
Low vitamin D is associated with lower activity of tyrosine hydroxylase
Vitamin D deficiency is common in Parkinson's Disease
Many studies have shown a high and increasing prevalence of vitamin D deficiency in the general population (Diehl and Chiu, 2010). Vitamin D deficiency is extremely prevalent in Kuwait (54% - Al-Mutairi etal, 2012), India (Babu and Calvo, 2010), Indonesia (45.5% of pregnant women - Ilmiawati etal, 2020), Europe (Brouwer etal, 2012); USA (Wentz etal, 2014; Forrest and Stuhldreher 2011), S. Korea (over 75% of females Park etal, 2020) and deficiency is higher in those with darker skin and during winter (Sawicki etal, 2016). More recently it has been calculated that over 80% of Americans are vitamin D deficient. Vitamin D deficiency is also common now in Australia and New Zealand (Shrapnel and Truswell, 2006; Quaggiotto etal, 2014).
Very few foods have significant levels of vitamin D, which is restricted mainly to fatty fish, beef liver, cheese, margarines, milk and eggs. Potentially this explains why vitamin D levels are significantly lower in vegetarians than non-vegetarians (Brooke etal, 1980). The increased incidence of vitamin D has been associated with the increasing use of sunscreens, long sleeves, following skin cancer campaigns. Using sunscreens with as little as a 15-factor protection factor protection cuts the skin's vitamin D production by 99 percent. There has also been a reduced consumption of foods such as salmon, tuna and mackerel, and vitamin D fortified dairy products such as milk, and a switch to such poor nutritional alternatives such as soy and almond detergent homogenized milk substitutes.
Whilst many are aware of the role of vitamin D in bone health, vitamin D has a unique role in brain development, including homeostasis, embyrogeneisis, neural differentiation, neurodevelopment, gene regulation and immunological modulation (Duan 2013). Vitamin D also has a role in neurotrophism, neuroprotection, and neuroplasticity (Cannell 2013), and vitamin D deficiency has been associated with developmental disorders and abnormal brain development in conditions such as autism (Eyles etal, 2013; 2009; Eissa etal, 2018; Wang etal, 2022). Vitamin D has also been shown to regulate the production of tyrosine hydroxylase. There is a significant association between low levels of vitamin D and the development of Parkinson's disease (
Luong and Nguyên 2012). Expression of tyrosine hydroxylase is required for maturation of dopamine-producing neurons (Pertile et al, 2016)Normal activation of vitamin D, is a well known process in which light from the sun, or more specifically UV light from the sun shines on the skin and causes the conversion of the precursor 7-dehydrocholesterol to be converted to vitamin D3 - cholecalciferol. This molecule then is further processed in the liver and converted to the inactive form 25-hydroxy-vitamin D. Finally the 25-hydroxyvitamin D (Calcidiol) is activated in the kidney to form 1,25di-hydroxyvitamin D (Calcitriol).
The brain is unique amongst the other organs in that it has its own enzyme, 1a-hydroxylase, that activates 25-hydroxyvitamin-D to the active form 1,25-dihydroxy-vitamin D. The active vitamin D so produced, then binds to specific vitamin D receptors in the brain, particularly in the hypothalamus, and dopaminergic neurons of the substantia nigra. High levels of expression of the 1-a-hydroxylase has been in the Purkinje cells in the cerebellum (Eyles etal, 2004). Malfunctioning Purkinje cells are directly associated with the reduced capacity for motor learning in children with autism. These cells are responsible for fine-tuned motor control, balance, proprioception, and the vestibulo-ocular reflex (VOR). The VOR is the reflex that stabilizes the eye movement during head turning, such that the eyes can still focus on a target, even when the head is turned.
Mode of activation of Vitamin D in the brain, following stimulation of the eye by 482 nm light.
Lack of vitamin D has also been associated with a loss in hippocampal volume (an area of the brain that regulates motivation, emotion, learning and memory), and hence low vitamin D would be associated with difficulty learning. Low vitamin D has been associated with cognitive decline in adults (Wentz etal, 2014). Low vitamin D in adults it has been associated with depression, Parkinson's disease and Alzheimer's disease Littlejohns etal, 2014; 2016; Dickens etal, 2011: Fullard and Duda, 2020). In experimental models, gestational vitamin D deficiency has been shown to cause permanent changes in the developing brains of rats (Levenson and Figueiroa, 2009; Feron etal, 2005), and has also been shown to lead to persistent changes in the adult brain (Feron etal, 2005; Eyles etal, 2012).
Interestingly vitamin D also promotes tyrosine hydroxylase (TH) and tryptophan hydroxylase 2 (TPH2) expression, AND results in a significant rise in monoamine oxidase A (MAOA) expression (Jianq 2014; Pertile 2016). This later finding is of considerable importance as MAOA is one of the only neurotransmitter related genes that are expressed on the X-chromosome, and hence alterations in MAO expression may provide the first reasonable hypothesis for the increased incidence of the condition in males, who by definition only have one X chromosome.
The importance of sun-exposure for the production of vitamin D has been known 1822 (nearly 200 years), and particularly exposure to UVB radiation (290-315 nm) (Holick 2006). However with the advent of sun-protection factors in the early 1870s, and the addition of high SPF value cosmetics and the increase in hours worked indoors, plus various sun-avoidance practices has seen a rise in the incidence of vitamin D deficiency, and an increase in the incidence of rickets with the result that vitamin D deficiency in children has once again reached epidemic proportions (Holick 2006). One of the potential sources of vitamin D is dairy, and so, the reduction in the consumption of dairy products, particularly those from free range cows and the switch to alternative products such as soy, and almond drinks, and adoption of a vegan diet can further reduce vitamin D levels.. Vitamin D deficiency is very common in some countries, and over 42% of Singapore residents (92), 45.5% of Saudi residents, and in 2018 over 82.5% of females in South Korea (an increase from 76% in 2008) were found to be vitamin D deficient. . Vitamin D status decreases with increases in weight.
In Australia, the effectiveness of the "Slip, Slop, Slap" campaign promoting sun-protection (starting in the late 1980s), was severely criticized as early as 2002 (Nowson etal, 2002), as at that stage the prevalence of vitamin D deficiency in women had already reached 23%, increasing the risk of osteoporosis, dementia, schizophrenia, Parkinson's disease, respiratory condition, diabetes, coronary disease, breast, and prostate cancer. In New Zealand as long ago as 2015, they were claiming the Slip, Slop, Slap campaign had gone too far. More recently the Australan Cancer council has added a warning to their web-site about vitamin D deficiency, thereby absolving themselves of blame. This is despite the fact that Parkinson's disease is the fastest growing condition in the world. It is almost unbelievable, that with all the references on low vitamin D and Parkinson's disease that any foundation can promote the "slip, slop, slap campaign", and in contrast not be promoting more sun exposure. The rate of growth of PD is over 4% compared with an annual population growth of 1.1%.Tyrosine Hydroxylase Deficiency in Parkinson's Disease
Parkinson's disease is a progressive condition that is postulated to be caused by lack of production of dopamine in the brain. It is likely that this lack is due to the lack of production of L-DOPA by the enzyme Tyrosine hydroxylase, as treatment of Parkinson's disease involves the administration of L-DOPA ."PD affects specifically TH-containing catecholamine neurons. The most marked neurodegeneration in patients with DA deficiency is observed in the nigro-striatal DA neurons, which contain abundant TH. Accordingly, TH has been speculated to play some important roles in the pathophysiology in PD" (Nagatsu et al, 2019). Production of tyrosine hydroxylase is stimulated by active vitamin D, and it has been shown that 1,25(OH)2D3 promotes the survival of dopaminergic neurons (Cui et al, 2015), and the activation of tyrosine hydroxylase, which is essential for survival of dopamine-producing neurons (Pertile et al, 2016). Vitamin D receptor is present in the substantia nigra, on the cells responsible for production of tyrosine hydroxylase (Cui et al, 2013), and vitamin D increases the expression of the tyrosine hydroxylase gene (Puchasz et al, 1996)
Iron Precipitation, alpha synuclein and Tyrosine Hydroxylase in Parkinson's Disease
Iron that has been taken up by neuronal cells is carried as Fe(III). This has to be reduced to the soluble form, Fe(II) inside the cell. Fe(II) can then be used in the synthesis of tyrosine hydroxylase, which subsequently produces L-DOPA. The enzyme responsible for reduction of Fe(III) to Fe(II) is alpha synuclein. Alpha synuclein mutations and differences in activity have been associated with severity of Parkinson's disease or with Fe(II) levels. Alpha synuclein functions as a ferrireductase, using copper and NADH as cofactors (Davies etal, 2011). copper appears to be important for both aggregation and cellular localisation of alpha-synuclein. Reduction in cellular copper resulted in a great decrease in aggregate formation both in terms of large aggregates visible in cells and oligomers observed in western blot analysis of cell extracts {Wang etal, 2010). Reduction in copper also resulted in a change in localisation of the protein which became more intensely localised to the plasma membrane in medium with low copperr. Alterred copper metabolism within the cell can lead to copper-induced cell death. This process is characterized by the abnormal accumulation of intracellular copper ions, leading to cellular dysfunction and eventual cell death (Pan et al, 2024). ron that precipitates within the cell is not available for use in the synthesis of iron-sulphur proteins, or ferroproteins such as tyrosine hydroxylase. The availability of Fe(II) is also controlled by levels of reduced glutathione (GSH). In functional vitamin B12 deficiency, levels of GSH are lower, and pyroglutamate is higher.
Iron Precipitation and Parkinson's Disease
Iron precipitation in the brain is a feature of neurodegenerative diseases such as Parkinson's Disease (Mezzaroba et al, 2019; Alverez Jerez et al, 2023; Behl et al, 2022; Hare and Double, 2016) There is evidence of iron deficiency as lower decreased Complex I activity.
Vitamin B12 and Parkinson's Disease
There is considerable evidence that vitamin B12 deficiency is implicated in PD.
-
Elevated homocysteine
-
Poor Sleep, which is associated with lower production of melatonin. Melatonin has been successfully tested in both in vitro and in vivo models of PD
-
Activation of the melatonin receptor MT1 prevents alph-syn-induced ferroptosis in PD
-
Low muscle strength - including low muscle strength. Lower production of creatine results in reduced muscle strength
-
OAT markers of deficiency - low GSH, elevated pyroglutamate, low CoQ10, elevated HVA, VMA, QA, KA, 5HIAA, low Tetrahydrobiopterin, elevated MMA, elevated branched chain amino acids, leucine, isoleucine, valine, alanine
-
Production of Acetylcholine involves the methylation of phosphatidylethanolamine to produce choline. Lack of methyl B12 activity results in the degeneration of the large cholinergic neurons of the PPN and Pars compacta,
-
Tau and Beta Amyloid pathologies. Folding of both Tau and Beta Amyloid requires disulfide exchange, which involves a correct balance of GSH:GSSG inside the cell. In functional B2/B12 deficiency, this range is changed, and this potentially will affect the structure of both proteins. In addition, any surface exposed free-thiol groups are then available for aggregation with other molecules of Tau or Beta-amyloid, thereby resulting in the Aggregated Tau and Beta-Amyloid pathologies (Kim etal, 2015:Saito, et al, 2021). In functional B2/B12 deficiency, the enzyme glutathione reductase cannot reduce GSSG, and so levels of free GSH are lower..
-
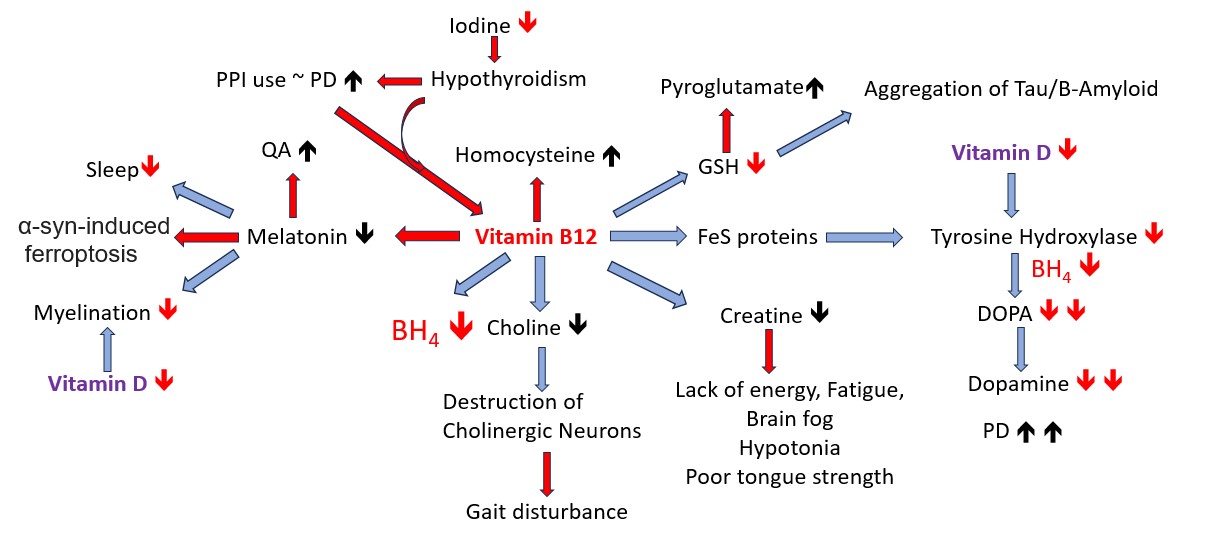
Proton Pump inhibitors and Parkinson's Disease
In functional B2 deficiency, there is lack of production of histamine in the stomach, which then stops the cephalic phase of digestion, and so may lead to gastric reflux. Identification of the deficiency is generally missed by the clinician who then prescribes proton pump inhibitors. These then give rise to iron and B12 deficiency. The results of a retrospective, nationwide, population-based cohort study in Tawian indicated that PPI use was associated with a higher risk of PD development. Functional B2 deficiency is common in hypothyroidism, and Hypothyroidism-associated parkinsonism may resemble idiopathic Parkinson's disease
Vitamin D Deficiency and Iron Deficiency
Several studies have shown an association between low iron and low vitamin D levels, presumably because iron is used in processing of vitamin D (Akermanns etal, 2017; in utero and in the new-born has been associated with delayed speech development (Hawes etal, 2015; Kamau etal, 2018; Malczewska-Lenczowska etal, 2018; Russell-Jones 2024).
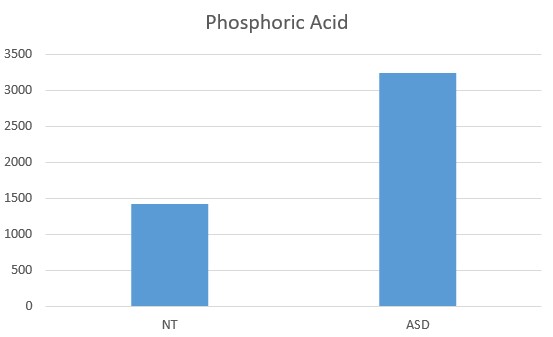
Altered Phosphate Metabolism in Parkinson's Disease
Active vitamin D is correlated with increased calcium by HMTA, as well as increased secretion of phosphoric acid . Low vitamin D is associated with decreased serum phosphate as is seen in early Parkinson's Disease (Håglin et al, 2016; 2020)
Recent studies have shown a dependency of functional B2, B12, and iron on activation of vitamin D. These findings have formed the basis for the Nexus TheoryTM
Russell-Jones, 2024 a, b
Changing the paradigm
Unfortunately too many cosmetic companies are earning billions of dollars from the sale of the high SPF cosmetics, so it is highly unlikely that they will change the formulations. It is also unlikely that while so many health professionals are making money out of treating vitamin D associated conditions such as autism, dementia, and Parkinson's disease, that they will change their strategy. Hence "“It is difficult to get a man to understand something when his salary
depends on his not understanding it.” (John Sinclair, 1932). Hence we have been unable to make progress with the cancer council in getting them to stop recommending the use of sun-protection products, nor several cosmetic companies. Hence it is not in the best interests of foundations getting millions of dollars in donations, for them to find a mode of prevention or cure for a condition that they are getting donations for. For instance the Australian Cancer Council (https://www.concer.org.au) has a major arm of its fund raising in selling SPF cosmetics, and clothing. This is despite countless publications on the protective effect of vitamin D and also how elevated vitamin D is protective against UVB mediated damage in the skin (Jagoca and Dixon, 2020; Song etal, 2012; Gupta etal, 2006).
Parkinson Disease has no known cause and so no known cure - the paradigm
"Everyday I have fewer reasons to live". This cannot be the inevitability of PD, hence, slowing or stopping the progress of the condition, or being able to reverse the symptoms is the most preferable outcome.. There is reason to be believe that the cause is known, and we have examples of individuals who have reversed the condition by addressing the deficiency in Selenium, vitamin B12 and vitamin D.
References
-
Asad etal, 2019 Developmental vitamin D deficiency produces behavioral phenotypes of relevance to autism in an animal model. Nutrients 11:1198
-
Duan, etal 2013 Relationship between vitamin D and autism spectrum disorder PMID 23965890
-
Cannell 2013 Autism, will vitamin D treat core symptoms? PMID 23725905
-
Eyles etal Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol 2013 34; 47-64 PMID 22796576
-
Forrest, K. Y., & Stuhldreher, W. L. (2011). Prevalence and correlates of vitamin D deficiency in US adults. Nutrition research (New York, N.Y.), 31(1), 48–54. https://doi.org/10.1016/j.nutres.2010.12.001
-
Eyles etal Developmental Vitamin D deficiency causes abnormal brain development. Psychoneuroendocrinol 2009 PMID 19500914
-
Ali etal Developmental vitamin D deficiency and autism:putative pathogenic mechanisms. J Steroid Biochem Mol Biol. 2018 PMID 28027915
-
Ali etal Developmental vitamin D deficiency produces behavioural phenotypes of relevance to autism in an animal model. Nutrients 2019 PMID 31137843
-
Eissa N., Al-Houqani M., Sadeq A., Ojha S. K., Sasse A., Sadek B. (2018). Current enlightenment about etiology and pharmacological treatment of autism spectrum disorder. Front. Neurosci. 12:304. 10.3389/fnins.2018.00304
-
Wang J, Huang H, Liu C, Zhang Y, Wang W, Zou Z, Yang L, He X, Wu J, Ma J, Liu Y. Research Progress on the Role of Vitamin D in Autism Spectrum Disorder. Front Behav Neurosci. 2022 May 10;16:859151. doi: 10.3389/fnbeh.2022.859151. PMID: 35619598; PMCID: PMC9128593.
-
Littlejohns etal. Vitamin D and dementia. J Prev Alzheimers Dis. 2016 : 3, 43-52
-
Littlejohns etal Vitamin D and the risk of dementia and Alzheimers disease Neurology 2014, 83, 920-928, PMID 21790207
-
Dickens etal Vitamin D, cognitive dysfunction and dementia in older adults. CNS Drugs 2011, 25, 629-639n Kesby etal 2009 Developmental vitamin D deficiency alters dopamine turnover in neonatal rat forebrain PMID 19500655
-
Kesby etal 2011 The effects of vitamin D on brain development and adult brain function. Mol Cell Endo 2011 347; 121-7 PMID 21664231
-
Fullard ME, Duda JE. A Review of the Relationship Between Vitamin D and Parkinson Disease Symptoms. Front Neurol. 2020 May 27;11:454. doi: 10.3389/fneur.2020.00454. PMID: 32536905; PMCID: PMC7267215.
-
Goh et al Mitochondrial dysfunction as a neurobiological subtype of Autism Spectrum Disorder.. JAMA psychiatry 2014 71: 665-671
-
Napoli et al Deficits in bioenergetics and impaired immune response in granulocytes from children with autism. Pediatrics 2014 133:e1405-10
-
Parikh et al A modern approach to the treatment of mitochondrial disease. Current Treatment Options in Neurology. 2009 11: 414-430
-
Bi et al. Prevalence of vitamin D deficiency in Singapore: Its implications to Cardiovascular Risk Factors. PMC4723156
-
Park et al. Vitamin D status in South Korean population... Medicine, 2018 97 Issue 26
-
Park HY, Lim YH, Park JB, Rhie J, Lee SJ. Environmental and Occupation Factors Associated with Vitamin D Deficiency in Korean Adults: The Korea National Health and Nutrition Examination Survey (KNHANES) 2010-2014. Int J Environ Res Public Health. 2020 Dec 8;17(24):9166. doi: 10.3390/ijerph17249166. PMID: 33302471; PMCID: PMC7762981.
-
Ali etal 2016 Developmental vitamin D deficiency and autism: Putative pathogenic mechanisms. PMID 28027915
-
Vinkhuyzen etal 2018 Gestational vitamin D deficiency and autism-related traits PMID 27895322
-
Chen etal 2016 Lower maternal serum 25(OH) D in first trimester associated with higher autism risk in Chinese offspring PMID 27663117
-
Garipardic etal, 2017 Association of Attention Deficit Hyperactivity Disorder and Autism Spectrum Disorders with mean platelet volume and vitamin D PMID 28319054
-
Ariganjoye, 2017 Pediatric hypovitaminosis D: Molecular perspectives and clinical implications PMID 28229097
-
Endres etal, 2016 Vitamin D deficiency in adult patients with schizophreniform and autism spectrum disorders PMID 27766084
-
Cannell and Grant 2013 What is the role of vitamin D in autism?. PMID 24494055
-
Grant and Soles 2009 Epidemiologic evidence supporting the role of maternal vitamin D as a risk factor for the development of infantile autism PMID 20592795
-
Kinney etal 2010 Environmental risk factors for autism: de they help cause de novo genetic mutations that contribute to the disorder. PMID 19699591
-
Shrapnel and Truswell 2006 VItamin D deficiency in Australia and New Zealand: What are the dietary options: Nutrition and Dietetics Wiley
-
Quaggiotto etal 2014 Vitamin D deficiency remains prevalent despite increased laboratory testing in New South Wales, Australia. Singapore Med J. PMID 24862752
-
DeLuca etal 2013 Review: the role of vitamin D in nervous system health and disease PMID 23336971
-
Jiang etal 2014 Neurochemical effects of chronic administration of calcitriol in rats PMID 25533012
-
Pertile etal, 2016 Vitamin D signaling and the differentiation of developing dopamine systems PMID 27450565
-
Basatemur et al. Trends in the diagnosis of vitamin D deficiency Pediatrics 2017 139 PMC5337117
-
Vitamin D supplementation in the UK https://www.breastfeedingnetwork.org.uk/vitamind/ https://www.nhs.uk/conditions/vitamins-and-minerals/vitamin-d/
-
Shibata et al. High prevalence of hypovitaminosis D in pregnant Japanese women with threatened premature delivery. J Bone Miner Metab 2011 29:615-20 PMID21384110
-
Nicolaidou et al. Low vitamin D status in mother-newborn pairs in Greece. Calcif Tissue Int 2006 78:337-42 PMID16830197
-
Haggarty etal. Vitamin D in pregnancy at high latitude in Scotland. Br J. Nutr 2013 109:898-905
-
Narchi etal, Longitudinal study of vitamin D status in the 1st 6 months of life. Ann Trop Paediatr. 2011 31:225-30
-
Ustuner etal. Maternal serum 25(OH)D levels in the third trimester of pregnancy during the winter season. Matern Fetal Neonatl Med 2011 24:1421-6
-
Viljakainen etal. Maternal vitamin D status determines bone variables in newborns. Endocrinol Metab. 2010 95: 1749-57
-
LowNesby-O'Dell etal Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age... Am J Clin Nutr. 2002 76: 1987-92
-
Pertile RA, Cui X, Eyles DW. Vitamin D signaling and the differentiation of developing dopamine systems. Neuroscience. 2016 Oct 1;333:193-203. doi: 10.1016/j.neuroscience.2016.07.020. Epub 2016 Jul 20. PMID: 27450565.
-
CMO Vitamin D supplements for at risk groups. dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_132508.pdf
-
Department of Health. Vitamin D an essential nutrient for all, but who is at risk of vitamin D deficiency? http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_111302.pdf
-
Henderson L, Irving K, Gregory J, et al. The National Diet and Nutrition Survey: adults aged 19 to 64 years. Vol 3. Vitamin and mineral intake and urinary analytes . London: Stationery Office, 2003: 1–160.
-
Hollis BW, Wagner CL. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. Am J Clin Nutr. 2004;80(Suppl 6):1752S–1758S
-
Hollis BW, Wagner CL, Howard CR, Ebeling M, Shary JR, Smith PG,Taylor SN, Morella K, Lawrence RA,Hulsey TC, Maternal Versus Infant Vitamin D Supplementation During Lactation: A Randomized Controlled Trial. Pediatrics 2015;136 (4):
-
Hawes, J. E., Tesic, D., Whitehouse, A. J., Zosky, G. R., Smith, J. T., & Wyrwoll, C. S. (2015). Maternal vitamin D deficiency alters fetal brain development in the BALB/c mouse. Behavioural brain research, 286, 192–200. https://doi.org/10.1016/j.bbr.2015.03.008
-
Akkermans, M. D., Eussen, S. R., van der Horst-Graat, J. M., van Elburg, R. M., van Goudoever, J. B., & Brus, F. (2017). A micronutrient-fortified young-child formula improves the iron and vitamin D status of healthy young European children: a randomized, double-blind controlled trial. The American journal of clinical nutrition, 105(2), 391–399. https://doi.org/10.3945/ajcn.116.136143
-
Kamau, M. W., Mirie, W., & Kimani, S. (2018). Compliance with Iron and folic acid supplementation (IFAS) and associated factors among pregnant women: results from a cross-sectional study in Kiambu County, Kenya. BMC public health, 18(1), 580. https://doi.org/10.1186/s12889-018-5437-2
-
Malczewska-Lenczowska, J., Sitkowski, D., Surała, O., Orysiak, J., Szczepańska, B., & Witek, K. (2018). The Association between Iron and Vitamin D Status in Female Elite Athletes. Nutrients, 10(2), 167. https://doi.org/10.3390/nu10020167
-
Holló, A., Clemens, Z., & Lakatos, P. (2014). Epilepsy and vitamin D. The International journal of neuroscience, 124(6), 387–393. https://doi.org/10.3109/00207454.2013.847836
-
Miratashi Yazdi, S. A., Abbasi, M., & Miratashi Yazdi, S. M. (2017). Epilepsy and vitamin D: a comprehensive review of current knowledge. Reviews in the neurosciences, 28(2), 185–201. https://doi.org/10.1515/revneuro-2016-0044
-
Specht, I. O., Thorsteinsdottir, F., Walker, K. C., Olsen, J., & Heitmann, B. L. (2020). Neonatal vitamin D status and risk of childhood epilepsy. Epilepsia, 61(6), 1282–1290. https://doi.org/10.1111/epi.16520
-
Jésus, P., Godet, B., Darthou-Pouchard, L., Fayemendy, P., Abdallah-Lebeau, F., Villeneuve, O., Marcon, C., Gimenez, L., Preux, P. M., Couratier, P., & Desport, J. C. (2020). Vitamin D status among patients with drug-resistant and non-drug-resistant epilepsy. International journal for vitamin and nutrition research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. Journal international de vitaminologie et de nutrition, 90(3-4), 205–209. https://doi.org/10.1024/0300-9831/a000459
-
Shellhaas, R. A., & Joshi, S. M. (2010). Vitamin D and bone health among children with epilepsy. Pediatric neurology, 42(6), 385–393. https://doi.org/10.1016/j.pediatrneurol.2009.12.005
Kija, E., Gidal, B. E., Shapson-Coe, A., Cader, S., van der Watt, G., Delport, S., & Wilmshurst, J. M. (2019). Vitamin D abnormalities and bone turn over analysis in children with epilepsy in the Western Cape of South Africa. Seizure, 69, 186–192. https://doi.org/10.1016/j.seizure.2019.04.020 -
Elmazny, A., Amer, H., Rashed, L., Khalil, S., & Magdy, R. (2020). Vitamin D status of untreated children and adolescent Egyptian patients with genetic generalized epilepsy: A case-control study. Epilepsy & behavior : E&B, 103(Pt A), 106840. https://doi.org/10.1016/j.yebeh.2019.106840
-
Mezzaroba L, Alfieri DF, Colado Simão AN, Vissoci Reiche EM. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology. 2019 Sep;74:230-241. doi: 10.1016/j.neuro.2019.07.007. Epub 2019 Aug 1. PMID: 31377220.
-
Alvarez Jerez P, Alcantud JL, de Los Reyes-Ramírez L, Moore A, Ruz C, Vives Montero F, Rodriguez-Losada N, Saini P, Gan-Or Z, Alvarado CX, Makarious MB, Billingsley KJ, Blauwendraat C, Noyce AJ, Singleton AB, Duran R, Bandres-Ciga S. Exploring the genetic and genomic connection underlying neurodegeneration with brain iron accumulation and the risk for Parkinson's disease. NPJ Parkinsons Dis. 2023 Apr 6;9(1):54. doi: 10.1038/s41531-023-00496-y. PMID: 37024536; PMCID: PMC10079978.
-
Behl T, Madaan P, Sehgal A, Singh S, Anwer MK, Makeen HA, Albratty M, Mohan S, Bungau S. Mechanistic Insights Expatiating the Redox-Active-Metal-Mediated Neuronal Degeneration in Parkinson's Disease. Int J Mol Sci. 2022 Jan 8;23(2):678. doi: 10.3390/ijms23020678. PMID: 35054862; PMCID: PMC8776156.
-
Hare DJ, Double KL. Iron and dopamine: a toxic couple. Brain. 2016 Apr;139(Pt 4):1026-35. doi: 10.1093/brain/aww022. PMID: 26962053.
-
Best CM, Riley DV, Laha TJ, Pflaum H, Zelnick LR, Hsu S, Thummel KE, Foster-Schubert KE, Kuzma JN, Cromer G, Larson I, Hagman DK, Heshelman K, Kratz M, de Boer IH, Hoofnagle AN. Vitamin D in human serum and adipose tissue after supplementation. Am J Clin Nutr. 2020 Nov 12;113(1):83–91. doi: 10.1093/ajcn/nqaa295. Epub ahead of print. PMID: 33184642; PMCID: PMC7779222.
-
Nagatsu T, Nakashima A, Ichinose H, Kobayashi K. Human tyrosine hydroxylase in Parkinson's disease and in related disorders. J Neural Transm (Vienna). 2019 Apr;126(4):397-409. doi: 10.1007/s00702-018-1903-3. Epub 2018 Jul 11. PMID: 29995172.
-
Cui X, Pertile R, Liu P, Eyles DW. Vitamin D regulates tyrosine hydroxylase expression: N-cadherin a possible mediator. Neuroscience. 2015 Sep 24;304:90-100. doi: 10.1016/j.neuroscience.2015.07.048. Epub 2015 Jul 23. PMID: 26210580.
-
Cui X, Pelekanos M, Liu PY, Burne TH, McGrath JJ, Eyles DW. The vitamin D receptor in dopamine neurons; its presence in human substantia nigra and its ontogenesis in rat midbrain. Neuroscience. 2013 Apr 16;236:77-87. doi: 10.1016/j.neuroscience.2013.01.035. Epub 2013 Jan 25. PMID: 23352937.
-
Puchacz E, Stumpf WE, Stachowiak EK, Stachowiak MK. Vitamin D increases expression of the tyrosine hydroxylase gene in adrenal medullary cells. Brain Res Mol Brain Res. 1996 Feb;36(1):193-6. doi: 10.1016/0169-328x(95)00314-i. PMID: 9011759.
-
Håglin L, Bäckman L. Covariation between plasma phosphate and daytime cortisol in early Parkinson's disease. Brain Behav. 2016 Oct 3;6(12):e00556. doi: 10.1002/brb3.556. PMID: 28031994; PMCID: PMC5166997.
-
Håglin L, Domellöf M, Bäckman L, Forsgren L. Low plasma thiamine and phosphate in male patients with Parkinson's disease is associated with mild cognitive impairment. Clin Nutr ESPEN. 2020 Jun;37:93-99. doi: 10.1016/j.clnesp.2020.03.012. Epub 2020 Apr 2. PMID: 32359763.
-
Davies P, Moualla D, Brown DR. Alpha-synuclein is a cellular ferrireductase. PLoS One. 2011 Jan 10;6(1):e15814. doi: 10.1371/journal.pone.0015814. Erratum in: PLoS One. 2011;6(1). doi: 10.1371/annotation/900a5247-7d03-4686-a544-5f7f64c0aac5. PMID: 21249223; PMCID: PMC3018422.
-
Wang X, Moualla D, Wright JA, Brown DR. Copper binding regulates intracellular alpha-synuclein localisation, aggregation and toxicity. J Neurochem. 2010 May;113(3):704-14. doi: 10.1111/j.1471-4159.2010.06638.x. Epub 2010 Feb 5. PMID: 20141569.
-
, , , et al. Cuproptosis: Mechanisms, biological significance, and advances in disease treatment—A systematic review. CNS Neurosci Ther. 2024; 30:e70039. doi:10.1111/cns.70039
-
Cobine PA, Brady DC. Cuproptosis: Cellular and molecular mechanisms underlying copper-induced cell death. Mol Cell. 2022 May 19;82(10):1786-1787. doi: 10.1016/j.molcel.2022.05.001. PMID: 35594843.; Cobine and Brady, 2022)
-
Madsen KH, Rasmussen LB, Andersen R, Mølgaard C, Jakobsen J, Bjerrum PJ, Andersen EW, Mejborn H, Tetens I. Randomized controlled trial of the effects of vitamin D–fortified milk and bread on serum 25-hydroxyvitamin D concentrations in families in Denmark during winter: the VitmaD study. Am J Clin Nutr. 2013 Aug;98(2):374-82. doi: 10.3945/ajcn.113.059469. PMID: 23783292.
Jagoda SV, Dixon KM. Protective effects of 1,25 dihydroxyvitamin D3 and its analogs on ultraviolet radiation-induced oxidative stress: a review. Redox Rep. 2020 Dec;25(1):11-16. doi: 10.1080/13510002.2020.1731261. PMID: 32093585; PMCID: PMC7054951.
Gupta R, Dixon KM, Deo SS, Holliday CJ, Slater M, Halliday GM, Reeve VE, Mason RS. Photoprotection by 1,25 dihydroxyvitamin D3 is associated with an increase in p53 and a decrease in nitric oxide products. J Invest Dermatol. 2007 Mar;127(3):707-15. doi: 10.1038/sj.jid.5700597. Epub 2006 Dec 14. PMID: 17170736.
Song EJ, Gordon-Thomson C, Cole L, Stern H, Halliday GM, Damian DL, Reeve VE, Mason RS. 1α,25-Dihydroxyvitamin D3 reduces several types of UV-induced DNA damage and contributes to photoprotection. J Steroid Biochem Mol Biol. 2013 Jul;136:131-8. doi: 10.1016/j.jsbmb.2012.11.003. Epub 2012 Nov 16. PMID: 23165145.
Nowson etal, Med J Aust 2002; 177: 149–52
Arshad R, Sameen A, Murtaza MA, Sharif HR, Iahtisham-Ul-Haq, Dawood S, Ahmed Z, Nemat A, Manzoor MF. Impact of vitamin D on maternal and fetal health: A review. Food Sci Nutr. 2022 Jul 7;10(10):3230-3240. doi: 10.1002/fsn3.2948. PMID: 36249984; PMCID: PMC9548347. Fiscaletti M, Stewart P, Munns CF. The importance of vitamin D in maternal and child health: a global perspective. Public Health Rev. 2017 Sep 1;38:19. doi: 10.1186/s40985-017-0066-3. PMID: 29450091; PMCID: PMC5809824
Sourander A, Upadhyaya S, Surcel HM, Hinkka-Yli-Salomäki S, Cheslack-Postava K, Silwal S, Sucksdorff M, McKeague IW, Brown AS. Maternal Vitamin D Levels During Pregnancy and Offspring Autism Spectrum Disorder. Biol Psychiatry. 2021 Dec 1;90(11):790-797. doi: 10.1016/j.biopsych.2021.07.012. Epub 2021 Jul 21. PMID: 34602240; PMCID: PMC8752030.
Saito T, Chiku T, Oka M, Wada-Kakuda S, Nobuhara M, Oba T, Shinno K, Abe S, Asada A, Sumioka A, Takashima A, Miyasaka T, Ando K. Disulfide bond formation in microtubule-associated tau protein promotes tau accumulation and toxicity in vivo. Hum Mol Genet. 2021 Oct 13;30(21):1955-1967. doi: 10.1093/hmg/ddab162. PMID: 34137825; PMCID: PMC8522637.
Kim D, Lim S, Haque MM, Ryoo N, Hong HS, Rhim H, Lee DE, Chang YT, Lee JS, Cheong E, Kim DJ, Kim YK. Identification of disulfide cross-linked tau dimer responsible for tau propagation. Sci Rep. 2015 Oct 15;5:15231. doi: 10.1038/srep15231. PMID: 26470054; PMCID: PMC4606741.
Cannell JJ. Vitamin D and autism, what's new? Rev Endocr Metab Disord. 2017 Jun;18(2):183-193. doi: 10.1007/s11154-017-9409-0. PMID: 28217829. Aagaard K, Møllegaard Jepsen JR, Sevelsted A, Horner D, Vinding R, Rosenberg JB, Brustad N, Eliasen A, Mohammadzadeh P, Følsgaard N, Hernández-Lorca M, Fagerlund B, Glenthøj BY, Rasmussen MA, Bilenberg N, Stokholm J, Bønnelykke K, Ebdrup BH, Chawes B. High-dose vitamin D3 supplementation in pregnancy and risk of neurodevelopmental disorders in the children at age 10: A randomized clinical trial. Am J Clin Nutr. 2024 Feb;119(2):362-370. doi: 10.1016/j.ajcnut.2023.12.002. Epub 2023 Dec 9. PMID: 38072183.
Russell-Jones, GJ. Altered Metabolism of Iron, Vitamin B, and Vitamin B12 Potentially Interfere with Vitamin D- Activation in Children with Autism. J Med - Clin Res & Rev. 2024a; 8(5): 1-6.
Russell-Jones GJ. Complex interaction between iron, vitamin B2, vitamin B12 and vitamin D during the activation of vitamin D. J Med - Clin Res & Rev. 2024b; 8(6); 1-5