
Methylation and Creatine
-
Creatine is an essential factor involved in ATP transport within the cytoplasm of the cell
-
Over 40% of S-Adenosyl-Methionine (SAM) produced in the body is involved in the production of Creatine.
-
Maintenance of methylation is critically dependent upon MethylCo(III)B12 and the enzyme methionine synthase (MTR)
-
The methylation cycle is also dependent upon two enzymes that require functional B2 sufficiency, MTHFR and MTRR
-
Functional deficiencies in vitamin B2, B6, B12 and folate can lead to reduced production of SAM, with consequent reduction in Creatine
-
Functional Vitamin B2 deficiency can be the result of dietary riboflavin, Iodine, Selenium or Molybdenum deficiency.
-
Deficiency of any of Iodine, Selenium, Molybdenum, vitamin B2, B12, B6 and folate in the womb can lead to a complete absence or strong deficiency of creatine in the brain of the embryo
-
Creatine deficiency syndromes, due to lack of creatine production in the brain include behavioural disorders, autistic behaviour, movement disorders, speech disorders, seizures and self-mutilation.
-
Reduced production of Creatine has been associated with many conditions including dementia, Parkinson's disease, Autism, Chronic Fatigue Syndrome.
Creatine and energy production within the cell
The penultimate step in energy production within the cell is the transfer of ATP across the mitochondrial membrane via the enzyme creatine-kinase, to an awaiting creatine molecule in the cytoplasm of the cell to make the high energy phosphate donor Creatine-Phosphate. Without this step, the generation of ATP within the mitochondria is futile. Thus, the creatine/phosphocreatine shuttle system is an essential component of transport of energy, produced in the mitochondria, into the cytoplasm of the cell (Sacks et al, 1978) . As such, it is thought to be essential for storing of high phosphate-bound energy, particularly in those cells with high energy demand. Creatine levels are high in tissues such as muscles, the brain, and are also very high in the oligodendrocytes Braissant etal, 2007; 2008; 2011) and astrocytes. It has been known for some time that Creatine-kinase mRNA levels are high in oligodendrocytes and astrocytes Molloy etal, 1992. The methylating enzyme GAMT, which is involved in the final step in creatine synthesis is similarly found in these cells Tachikawa etal, 2004). Whist originally it was thought that most of the Creatine in the brain was of peripheral origin, more recently evidence suggests that the ability of creatine to cross the blood brain barrier is very poor, and hence the majority of Creatine used in the brain comes from endogenous synthesis (Braissant etal, 2007; 2008; 2011). This, then, potentially creates a problem in functional vitamin B12 deficiency, because the synthesis of Creatine in the brain will also require an active methylation cycle locally in the brain, to provide the methyl donor SAM for use by GAMT in the synthesis of Creatine.
Modified from Beard and Braissant, 2010.
CK - Creatine Kinase mCK - mitochondrial Creatine Kinase, CAC - citric acid cycle, AGAT - Arginine-Glycine AmidinoTransferase, GAMT - GaunidinoAcetate-MethylTransferase, SAM - S-Adenosylmethionine, AcCoA - AcetylCoA.
Synthesis of Creatine
Synthesis of creatine occurs in two steps
1. Conjugation of glycine, and arginine, via the enzyme, l-arginine:glycine amidinotransferase (AGAT), to produce guanidinoacetate acid (GAA),
2. Methylation of Guanidinoacetate by the enzyme Guanidinoacetate-N-Methyl transferase (GNMT, GAMT)
The requirement of GAMT/GNMT for SAM, means that there is a co-requirement for an effective methylation cycle, which in turn requires sufficient active MethylCo(III)B12. Hence in functional B12 deficiency, you will also have functional deficiency in the GAMT/GNMT enzyme, and will therefore have reduced production of creatine. A deficiency of vitamin B12 in the peripheral organs is relatively easy to fix, BUT, loading a deficient brain with vitamin B12 is much harder.
Levels of Creatine Kinase expression vary between cells in the brain, with levels 17-fold higher in oligodendrocytes, and 14-fold higher in astrocytes than in normal neurons (Molloy etal, 1992). Olidogendrocytes are the main source of endogenously synthesised creatine, and loss or lowering of creatine synthesis leads to delayed myelination, and lead to intellectual delays (seen in absolute B12 deficiency), seizures, and autistic behaviour (Rosko et al, 2023)
Deficiency of GAMT/GNMT activity and Autism
Lack of activity of the enzyme GAMT has been shown to give rise to many of the symptoms of autism. In addition lack of activity of GAMT leads to prolonged fatigue, similar to that in Chronic Fatigue Syndrome. Lack of activity of GAMT enzyme is associated with many symptoms associated with Autism and Alzheimer's Disease. In children GAMT deficiency can cause severe developmental and mental retardation, speech delay, recurrent seizures (and TICS), behavioral changes, and movement disorders, including Muscular hypotonia, mild spasticity, and coordination disturbances (Braissant etal, 2011; Longo etal, 2011; Pacheva etal, 2016; Stöckler et al, 1994; Mercimek-Mahmutoglu et al, 2006; Stockler-Ipsiroglu et al, 2014; Mercimek-Mahmutoglu et al 2014; O'Rourke et al, 2009; Araújo et al, 2005; Lion-François et al, 2006; Mercimek-Mahmutoglu et al, 2009; Leuzzi et al, 2013 Schulze et al, 2006;Verbruggen et al 2007; Morris et al, 2007; Item etal, 2004.
Lack of methylation, due to functional methyl B12 deficiency, can lead to toxic build up of Guandinoacetate in the brain, which can in turn lead to symptoms of epilepsy. Deficiency of activity of GAMT leads to greatly decreased levels of Creatine in the brain (Braissant et al, 2011; 2008) and CNS which is the main organ affected by Creatine deficiency (Stöckleretal, 1994; Schulze et al, 1997; Schulze and Battini 2007; Salomons et al, 2001).
Despite the inextricable linkage between methyl B12, the methylation cycle, the production of SAM, and the need for GAMT to use SAM in the final step in the production of Creatine, not one review on GAMT and creatine production, that we could find ever cited it, and in fact ever mentioned vitamin B12. This is despite the frequent association between vitamin B12 deficiency and conditions such as autism, AD, and CFS!!
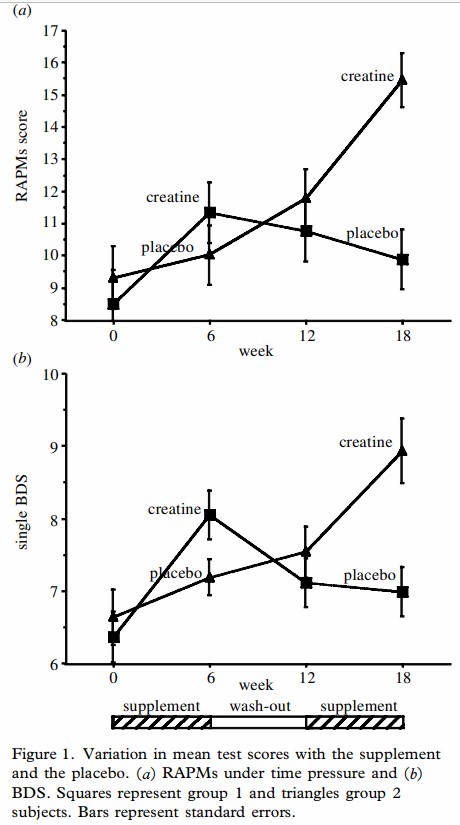
Creatine supplementation and Improvement in Cognition
Studies on Vegan subjects given 5 gm per day creatine-monohydrate, showed a significant improvement in cognitive scores after 4 weeks of supplementation. The mechanism was presumed to be greater uptake of creatine into the brain and neuronal cells (Rae etal, 2003). A similar improvement was seen cognition by Hammet and co-workers (2010). See review by Candow (2023)
Methylation, Neurodevelopment and Autism
Apart from the obvious requirement of Methyl B12 for energy production in oligodendrocytes, methylB12 has a secondary role in the production of melatonin. Thus, Melatonin, together with vitamin D, stimulates neuronal stem cells to differentiate into oligodendrocytes, which are the cells in the brain that are responsible for myelination of the nerves in the brain. Hence a deficiency in methyl B12 would result in lower stimulation of differentiation of neuronal stem cells into oligodendrocytes, and lack of production of creatine, would in turn lead to lower energy within the oligodendrocytes, lower myelination and a greater chance of developmental and mental retardation.
Dietary supplements that may help increase creatine, Neurodevelopment and Autism
The last step in creatine synthesis involves SAM, which is a conjugate of Adenosine and Methionine. Potentially foods high in methionine may aid in increasing levels of SAM, such as dried egg whites, dried spirulina, lean beef, brazil nuts, lean lamb, bacon, parmesan cheese, chicken breast and tuna. Of note, in functional B12 deficiency, the continued use of dietary methionine to supply SAM will eventually lead to high levels of homocysteine. Whilst the majority of those on an omnivore diet obtain around half their daily creatine requirements from red meat, those on a vegan or vegetarian diet obtain very little from their food and so must rely on local synthesis (da Silve etal, 2009). Dietary creatine has limitations in the very little of the material crosses the blood brain barrier.
References
Saks, V. A., Rosenshtraukh, L. V., Smirnov, V. N., & Chazov, E. I. (1978). Role of creatine phosphokinase in cellular function and metabolism. Canadian journal of physiology and pharmacology, 56(5), 691–706. https://doi.org/10.1139/y78-113
Tachikawa, M., Kasai, Y., Yokoyama, R., Fujinawa, J., Ganapathy, V., Terasaki, T., & Hosoya, K. (2009). The blood-brain barrier transport and cerebral distribution of guanidinoacetate in rats: involvement of creatine and taurine transporters. Journal of neurochemistry, 111(2), 499–509. https://doi.org/10.1111/j.1471-4159.2009.06332.x
Beard, E. and Braissant, O. (2010) Synthesis and transport of creatine in the CNS: importance for cerebral functions. J Biochem 115: 297-313
Braissant, O., Bachmann, C., & Henry, H. (2007). Expression and function of AGAT, GAMT and CT1 in the mammalian brain. Sub-cellular biochemistry, 46, 67–81. https://doi.org/10.1007/978-1-4020-6486-9_4
Mercimek-Andrews, S., & Salomons, G. S. (2009). Creatine Deficiency Syndromes. In M. P. Adam (Eds.) et. al., GeneReviews®. University of Washington, Seattle.
Joncquel-Chevalier Curt, M., Voicu, P. M., Fontaine, M., Dessein, A. F., Porchet, N., Mention-Mulliez, K., Dobbelaere, D., Soto-Ares, G., Cheillan, D., & Vamecq, J. (2015). Creatine biosynthesis and transport in health and disease. Biochimie, 119, 146–165. https://doi.org/10.1016/j.biochi.2015.10.022
Braissant, O., & Henry, H. (2008). AGAT, GAMT and SLC6A8 distribution in the central nervous system, in relation to creatine deficiency syndromes: a review. Journal of inherited metabolic disease, 31(2), 230–239. https://doi.org/10.1007/s10545-008-0826-9
Braissant, O., Henry, H., Béard, E., & Uldry, J. (2011). Creatine deficiency syndromes and the importance of creatine synthesis in the brain. Amino acids, 40(5), 1315–1324. https://doi.org/10.1007/s00726-011-0852-z
Roschel, H., Gualano, B., Ostojic, S. M., & Rawson, E. S. (2021). Creatine Supplementation and Brain Health. Nutrients, 13(2), 586. https://doi.org/10.3390/nu13020586
Degrauw, T. J., & Jakobs, C. (2001). X-linked creatine-transporter gene (SLC6A8) defect: a new creatine-deficiency syndrome. American journal of human genetics, 68(6), 1497–1500. https://doi.org/10.1086/320595
Stöckler, S., Holzbach, U., Hanefeld, F., Marquardt, I., Helms, G., Requart, M., Hänicke, W., & Frahm, J. (1994). Creatine deficiency in the brain: a new, treatable inborn error of metabolism. Pediatric research, 36(3), 409–413. https://doi.org/10.1203/00006450-199409000-00023
Schulze, A., & Battini, R. (2007). Pre-symptomatic treatment of creatine biosynthesis defects. Sub-cellular biochemistry, 46, 167–181. https://doi.org/10.1007/978-1-4020-6486-9_9
Schulze, A., Hess, T., Wevers, R., Mayatepek, E., Bachert, P., Marescau, B., Knopp, M. V., De Deyn, P. P., Bremer, H. J., & Rating, D. (1997). Creatine deficiency syndrome caused by guanidinoacetate methyltransferase deficiency: diagnostic tools for a new inborn error of metabolism. The Journal of pediatrics, 131(4), 626–631. https://doi.org/10.1016/s0022-3476(97)70075-1
Molloy, G. R., Wilson, C. D., Benfield, P., de Vellis, J., & Kumar, S. (1992). Rat brain creatine kinase messenger RNA levels are high in primary cultures of brain astrocytes and oligodendrocytes and low in neurons. Journal of neurochemistry, 59(5), 1925–1932. https://doi.org/10.1111/j.1471-4159.1992.tb11028.x
Tachikawa, M., Fukaya, M., Terasaki, T., Ohtsuki, S., & Watanabe, M. (2004). Distinct cellular expressions of creatine synthetic enzyme GAMT and creatine kinases uCK-Mi and CK-B suggest a novel neuron-glial relationship for brain energy homeostasis. The European journal of neuroscience, 20(1), 144–160. https://doi.org/10.1111/j.1460-9568.2004.03478.x
Cameron, J. M., Levandovskiy, V., Roberts, W., Anagnostou, E., Scherer, S., Loh, A., & Schulze, A. (2017). Variability of Creatine Metabolism Genes in Children with Autism Spectrum Disorder. International journal of molecular sciences, 18(8), 1665. https://doi.org/10.3390/ijms18081665
Leuzzi, V., Mastrangelo, M., Battini, R., & Cioni, G. (2013). Inborn errors of creatine metabolism and epilepsy. Epilepsia, 54(2), 217–227. https://doi.org/10.1111/epi.12020
Burbaeva, G., Savushkina, O. K., & Dmitriev, A. D. (1999). Mozgovaia izoforma kreatinfosfokinazy v norme i pri psikhicheskikh zabolevaniiakh (bolezn' Al'tsgemera, shizofreniia) [Brain isoforms of creatine kinase in health and mental diseases: Alzheimer's disease and schizophrenia]. Vestnik Rossiiskoi akademii meditsinskikh nauk, (1), 20–24.
Chen et al, Cretinism revisited. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 39–50.
Rajatanavin et al. Endemic cretinism in Thailand: a multidiciplinary study. Eur. J. Endocrinol. 1997, 137, 349–355.
da Silva, R. P., Nissim, I., Brosnan, M. E., & Brosnan, J. T. (2009). Creatine synthesis: hepatic metabolism of guanidinoacetate and creatine in the rat in vitro and in vivo. American journal of physiology. Endocrinology and metabolism, 296(2), E256–E261. https://doi.org/10.1152/ajpendo.90547.2008
Rosko LM, Gentile T, Smith VN, Manavi Z, Melchor GS, Hu J, Shults NV, Albanese C, Lee Y, Rodriguez O, Huang JK. Cerebral Creatine Deficiency Affects the Timing of Oligodendrocyte Myelination. J Neurosci. 2023 Feb 15;43(7):1143-1153. doi: 10.1523/JNEUROSCI.2120-21.2022. Epub 2023 Feb 2. PMID: 36732069; PMCID: PMC9962777.
Rae C, Digney AL, McEwan SR, Bates TC. Oral creatine monohydrate supplementation improves brain performance: a double-blind, placebo-controlled, cross-over trial. Proc Biol Sci. 2003 Oct 22;270(1529):2147-50. doi: 10.1098/rspb.2003.2492. PMID: 14561278; PMCID: PMC1691485.
Hammett ST, Wall MB, Edwards TC, Smith AT. Dietary supplementation of creatine monohydrate reduces the human fMRI BOLD signal. Neurosci Lett. 2010 Aug 2;479(3):201-5. doi: 10.1016/j.neulet.2010.05.054. Epub 2010 May 24. PMID: 20570601. https://link.springer.com/article/10.1007/s40279-023-01870-9?fromPaywallRec=true
Copyright © 2021 B12 Oils. All Rights Reserved.
Reproduction in whole or in part in any form or medium without express written
permission is prohibited